
How many positional isomers are there in ${{\text{C}}_5}{{\text{H}}_{11}}Br$ ?
Answer
502.2k+ views
Hint: To answer this question we should learn about isomers first. Isomerism is a process in which compounds have different chemical structures but the same chemical formula. A Chemical compound that has the same chemical formula but a different chemical structure is known as an isomer.
Complete answer:
Isomers are of various types, positional isomers, chain isomers, functional isomers, and Stereoisomers, based on the orientation of the compound in three-dimensional space. Ther are divided into two categories, Geometric isomers, and Optical isomers.
In this question we are required to find the positional isomers of ${{\text{C}}_5}{{\text{H}}_{11}}Br$ or $1 - Bromopentane$
Let’s see what positional isomers are.
Positional Isomers: These are constitutional isomers that have the same carbon skeleton and same functional groups but they differ in the position of the functional group attached to the carbon chain. It involves the attachment of functional groups of different carbon atoms in the carbon chain.
Let see the structure of $1 - Bromopentane$
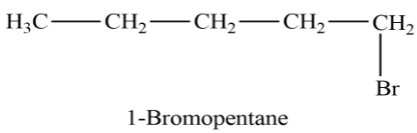
In this structure, we can see that the functional group Bromine is attached to the first carbon in the carbon chain.
now let’s see an isomer
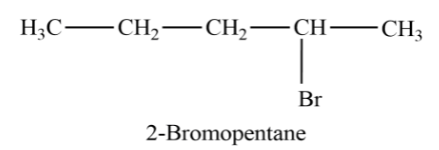
In the above structure, we can see that the Functional group is attached to the second carbon in the carbon chain. So, the position of the functional group has changed and the chemical formula is still the same.
Let’s see another isomer
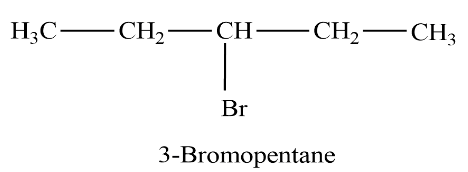
In this structure, we can see that the Functional group is attached to the third carbon in the carbon chain. So, the position of the functional group has changed and the chemical formula remains the same.
So, the given compound has three positional isomers.
Note:
The compound given has only three positional isomers because IUPAC naming is done according to some rules, one of those rules is that numbering of carbon starts from that carbon to which the functional group is attached so if we see the last structure if we change the position of Bromine again it will give the same isomers like the first two structures.
Complete answer:
Isomers are of various types, positional isomers, chain isomers, functional isomers, and Stereoisomers, based on the orientation of the compound in three-dimensional space. Ther are divided into two categories, Geometric isomers, and Optical isomers.
In this question we are required to find the positional isomers of ${{\text{C}}_5}{{\text{H}}_{11}}Br$ or $1 - Bromopentane$
Let’s see what positional isomers are.
Positional Isomers: These are constitutional isomers that have the same carbon skeleton and same functional groups but they differ in the position of the functional group attached to the carbon chain. It involves the attachment of functional groups of different carbon atoms in the carbon chain.
Let see the structure of $1 - Bromopentane$
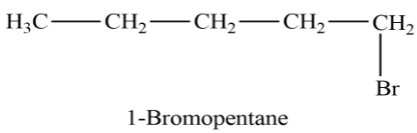
In this structure, we can see that the functional group Bromine is attached to the first carbon in the carbon chain.
now let’s see an isomer
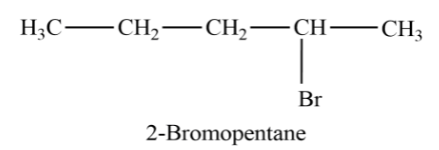
In the above structure, we can see that the Functional group is attached to the second carbon in the carbon chain. So, the position of the functional group has changed and the chemical formula is still the same.
Let’s see another isomer

In this structure, we can see that the Functional group is attached to the third carbon in the carbon chain. So, the position of the functional group has changed and the chemical formula remains the same.
So, the given compound has three positional isomers.
Note:
The compound given has only three positional isomers because IUPAC naming is done according to some rules, one of those rules is that numbering of carbon starts from that carbon to which the functional group is attached so if we see the last structure if we change the position of Bromine again it will give the same isomers like the first two structures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




