
What is polymerization? Give an example of a polymerization reaction.
Answer
522.9k+ views
Hint: The monomer is the smallest unit of the polymer molecule. The polymer is the biggest molecule which contains repeating units of the monomers (small units). In nature all the polymers are made up of smaller units called monomers.
Complete answer:
- In the question it is asked to write about polymerization with an example.
- All the polymers in nature are going to be prepared through a process called polymerization.
- The monomeric units combine with other monomers or with the same monomers through a process called polymerization.
- There are a lot of different polymerization methods available to prepare polymers from monomers.
- The type of polymerization technique is going to depend on the nature of the monomers.
- The different polymerization methods are
1) Addition polymerization method
2) Condensation polymerization method
- Here we can discuss the preparation of the terylene polymer and it is as follows.
Terylene is an example of a co-polymer.
- Means terylene is going to contain two different monomeric units.
- The monomeric units which are present in the terylene polymer are ethylene glycol and terephthalic acid.
- The other name of terephthalic acid is 1,4-benzene dicarboxylic acid.
- The structure of ethylene glycol and terephthalic acid are as follows.
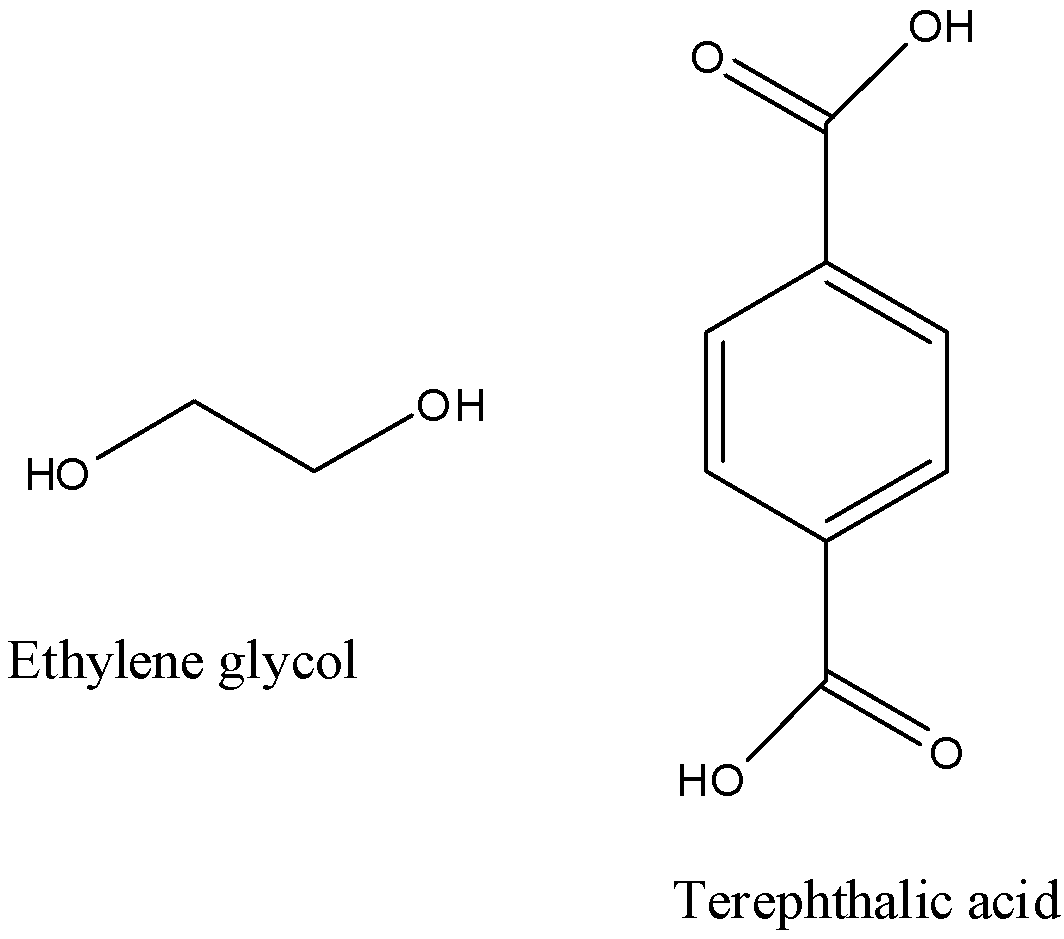
- The polymer which is formed by the polymerization of ethylene glycol and terephthalic acid is as follows.
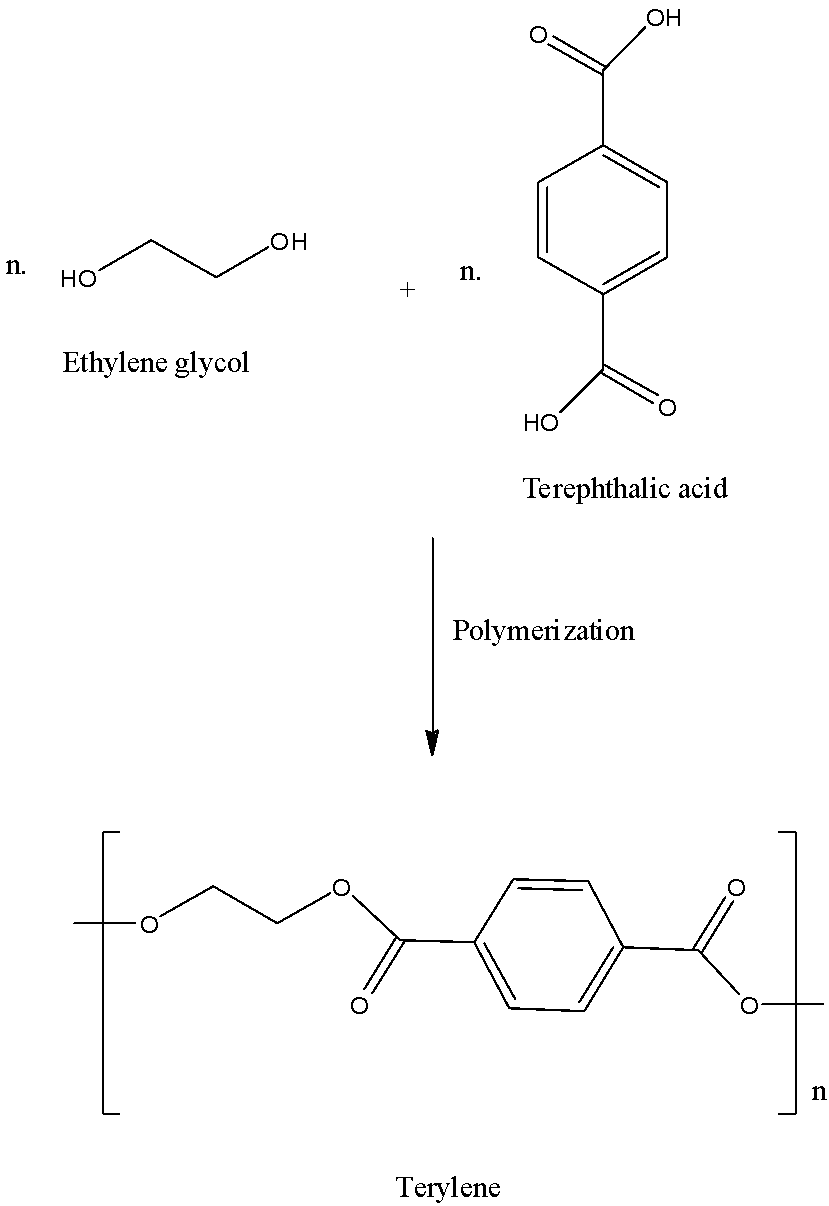
- We can see the structure of the terylene polymer in the above polymerization method called condensation polymerization and it contains the ethylene glycol and terephthalic acid.
Note:
The type of functional groups present in the monomeric units is going to decide the type of polymerization method they undergo. If there is a presence of unsaturated functional groups in the monomers then they will undergo addition polymerization.
Complete answer:
- In the question it is asked to write about polymerization with an example.
- All the polymers in nature are going to be prepared through a process called polymerization.
- The monomeric units combine with other monomers or with the same monomers through a process called polymerization.
- There are a lot of different polymerization methods available to prepare polymers from monomers.
- The type of polymerization technique is going to depend on the nature of the monomers.
- The different polymerization methods are
1) Addition polymerization method
2) Condensation polymerization method
- Here we can discuss the preparation of the terylene polymer and it is as follows.
Terylene is an example of a co-polymer.
- Means terylene is going to contain two different monomeric units.
- The monomeric units which are present in the terylene polymer are ethylene glycol and terephthalic acid.
- The other name of terephthalic acid is 1,4-benzene dicarboxylic acid.
- The structure of ethylene glycol and terephthalic acid are as follows.

- The polymer which is formed by the polymerization of ethylene glycol and terephthalic acid is as follows.

- We can see the structure of the terylene polymer in the above polymerization method called condensation polymerization and it contains the ethylene glycol and terephthalic acid.
Note:
The type of functional groups present in the monomeric units is going to decide the type of polymerization method they undergo. If there is a presence of unsaturated functional groups in the monomers then they will undergo addition polymerization.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




