
How can polarity of molecules be predicted from their geometry?
Answer
547.5k+ views
Hint: To predict the polarity of a molecule from its geometry, first we have to know about the electronegativity of each atom present in that molecule and type of bond between two atoms to ensure the structure of given molecule.
Complete answer:
Polarity of a molecule should be predicted from their geometry in the following manner:
-First of all we have to be familiar about the electronegativity of each atom which is present in the given molecule.
-When all the atoms with charges are present in a symmetrically manner inside the molecule then they show non – polar behavior and when the atoms with charges are located unsymmetrically then they show polar behavior.
-let us consider a molecule of water i.e. ${{\text{H}}_{\text{2}}}{\text{O}}$. In this molecule oxygen is more electronegative as compare to two hydrogen atoms and bear a partial negative charge on it and hydrogen atoms bear a positive charge on itself, and their geometry or structure is shown as follow:
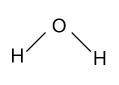
In this molecule positive charge is not symmetrically present around the central atom i.e. on one side of the molecule more positive charge is present as compared to the other side, as a result of which water molecule is polar in nature.
-In the ${\text{B}}{{\text{F}}_{\text{3}}}$ molecule, fluorine bears a partial negative charge and boron bear a partial positive charge and their geometry is shown as follow:
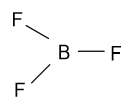
In the above molecule all fluorine atoms with partial negative charge are present symmetrically around the central atom, as a result of which it is polar in nature.
So, by the following description we will predict the polarity of a molecule by its geometry.
Note:
For predicting the polarity of a molecule we have to know each and every spatial arrangement of bonds present in a molecule and for this we should know about the hybridization of the central atom of a molecule.
Complete answer:
Polarity of a molecule should be predicted from their geometry in the following manner:
-First of all we have to be familiar about the electronegativity of each atom which is present in the given molecule.
-When all the atoms with charges are present in a symmetrically manner inside the molecule then they show non – polar behavior and when the atoms with charges are located unsymmetrically then they show polar behavior.
-let us consider a molecule of water i.e. ${{\text{H}}_{\text{2}}}{\text{O}}$. In this molecule oxygen is more electronegative as compare to two hydrogen atoms and bear a partial negative charge on it and hydrogen atoms bear a positive charge on itself, and their geometry or structure is shown as follow:

In this molecule positive charge is not symmetrically present around the central atom i.e. on one side of the molecule more positive charge is present as compared to the other side, as a result of which water molecule is polar in nature.
-In the ${\text{B}}{{\text{F}}_{\text{3}}}$ molecule, fluorine bears a partial negative charge and boron bear a partial positive charge and their geometry is shown as follow:

In the above molecule all fluorine atoms with partial negative charge are present symmetrically around the central atom, as a result of which it is polar in nature.
So, by the following description we will predict the polarity of a molecule by its geometry.
Note:
For predicting the polarity of a molecule we have to know each and every spatial arrangement of bonds present in a molecule and for this we should know about the hybridization of the central atom of a molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




