
Polarity in a molecule and hence the dipole moment depends primarily on the shape of the molecule and electronegativity of the constituent atoms. Which of the molecules has the highest dipole moment?
A. $C{O_2}$ \[\]
B. $ HI $
C. ${H_2}O$
D. $S{O_2}$
Answer
585.6k+ views
Hint: dipole moment of a bond A-B is charge accumulated on either A and B without sign multiplied with the length of bond A-B.
Complete step by step solution: Let's analyze the options one by one, $C{O_2}$ is a triatomic molecule where there is a slight difference between the electronegativity of C and O that is oxygen is more electronegative than carbon but the structure of $C{O_2} $ is linear. One carbon atom joins the two oxygen atoms with a double bond with each of them and makes a 180 degree angle overall. Therefore the dipole moment thus observed along the direction of carbon to oxygen atoms on both the sides cancel each other making the net dipole moment zero.
HI is a diatomic molecule where there is high electronegativity difference between hydrogen and iodine as iodine is a group 17 member group of highest electronegativity elements. But the electronegativity in the group decreases as one moves down the group hence iodine is the least electronegative element and its electronegativity is less than oxygen.
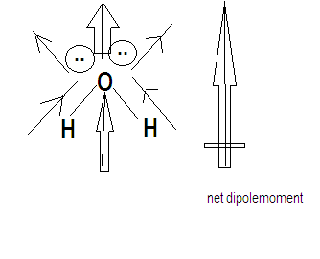
${H_2}O$ is a triatomic molecule with an angular structure. Oxygen is more electronegative than hydrogen. Both the lone pair and the dipole moment of both the O-H bonds contribute in one direction which is why ${H_2}O$ has a maximum dipole moment .
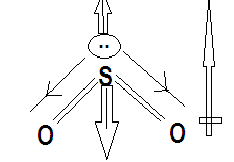
$S{O_2}$ is also a triatomic molecule but sulphur and oxygen does not have a high electronegativity difference. Its dipole moment is reduced by difference in the direction of dipole moment contributed by the double bond and lone pair.
Hence the correct option is option C.
Note: For diatomic heteroatomic molecules, the bond moment is equal to the dipole moment which is zero as there is no shifting of bond pair towards any atom.
Complete step by step solution: Let's analyze the options one by one, $C{O_2}$ is a triatomic molecule where there is a slight difference between the electronegativity of C and O that is oxygen is more electronegative than carbon but the structure of $C{O_2} $ is linear. One carbon atom joins the two oxygen atoms with a double bond with each of them and makes a 180 degree angle overall. Therefore the dipole moment thus observed along the direction of carbon to oxygen atoms on both the sides cancel each other making the net dipole moment zero.
HI is a diatomic molecule where there is high electronegativity difference between hydrogen and iodine as iodine is a group 17 member group of highest electronegativity elements. But the electronegativity in the group decreases as one moves down the group hence iodine is the least electronegative element and its electronegativity is less than oxygen.
${H_2}O$ is a triatomic molecule with an angular structure. Oxygen is more electronegative than hydrogen. Both the lone pair and the dipole moment of both the O-H bonds contribute in one direction which is why ${H_2}O$ has a maximum dipole moment .

$S{O_2}$ is also a triatomic molecule but sulphur and oxygen does not have a high electronegativity difference. Its dipole moment is reduced by difference in the direction of dipole moment contributed by the double bond and lone pair.

Hence the correct option is option C.
Note: For diatomic heteroatomic molecules, the bond moment is equal to the dipole moment which is zero as there is no shifting of bond pair towards any atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




