
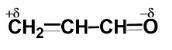
Polarisation of electrons in acrolein may be written as:
A.
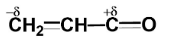
B.
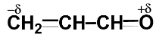
C.
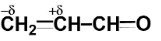
D.
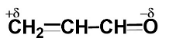
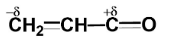
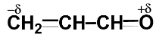
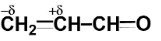

Answer
533.4k+ views
Hint: The electronegative atom always tends to carry a partial negative charge while the less electronegative atom tends to carry a partial positive charge. The electropositive atom cannot possibly acquire a negative charge.
Complete answer:
Polarisation is a phenomenon of acquiring a partial positive or partial negative charge due to the difference in the electronegativity of any two bonding atoms.
Electronegativity is the tendency of an atom to attract a bonding pair of electrons towards itself. During the bond formation, if the two atoms differ even slightly in their electronegativity, then the electron will be attracted more toward the more electronegative atom and that atom will then acquire a partial negative charge. On the other hand, the electron has now been shifted a little to the opposite of a less electronegative atom, so it will acquire a partial positive charge.
In this way, polarisation happens and we can easily tell by looking at the electronegativity trend that which atom in the compound will be partially positive or negative charged.
Similarly, in acrolein, we have carbon atoms and an oxygen atom. Since the electronegativity of all carbon atoms is the same, therefore there will not be any partial charge on adjacent carbon atoms.
Since oxygen is more electronegative than carbon, so oxygen will carry a partial negative charge while carbon will carry a partial positive charge.
Additional information: Acrolein is an unsaturated aldehyde that produces the smell of burnt fat when glycerol breakdowns into acrolein.
Hence, the correct answer is:
(D)
Note: If the electronegativity difference between two atoms is very high then they form an ionic compound by completely transferring the electrons between each other.
Complete answer:
Polarisation is a phenomenon of acquiring a partial positive or partial negative charge due to the difference in the electronegativity of any two bonding atoms.
Electronegativity is the tendency of an atom to attract a bonding pair of electrons towards itself. During the bond formation, if the two atoms differ even slightly in their electronegativity, then the electron will be attracted more toward the more electronegative atom and that atom will then acquire a partial negative charge. On the other hand, the electron has now been shifted a little to the opposite of a less electronegative atom, so it will acquire a partial positive charge.
In this way, polarisation happens and we can easily tell by looking at the electronegativity trend that which atom in the compound will be partially positive or negative charged.
Similarly, in acrolein, we have carbon atoms and an oxygen atom. Since the electronegativity of all carbon atoms is the same, therefore there will not be any partial charge on adjacent carbon atoms.
Since oxygen is more electronegative than carbon, so oxygen will carry a partial negative charge while carbon will carry a partial positive charge.
Additional information: Acrolein is an unsaturated aldehyde that produces the smell of burnt fat when glycerol breakdowns into acrolein.
Hence, the correct answer is:
(D)

Note: If the electronegativity difference between two atoms is very high then they form an ionic compound by completely transferring the electrons between each other.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




