
How do polar molecules form hydrogen bonds?
Answer
555.3k+ views
Hint: Hydrogen bonding is a special sort of dipole-dipole attraction among molecules,It results from the attractive force among a hydrogen atom covalently bonded to a very electronegative atom such as a N, O, or F atom and some other very electronegative atom.
Complete step by step answer:
Hydrogen bonds can be intermolecular (happening among separate molecules) or intramolecular (going on among parts of the identical molecule). Depending on the character of the donor and acceptor atoms which constitute the bond, their geometry, and surroundings.
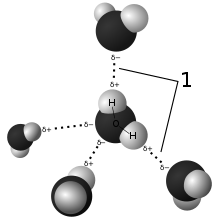
In chemistry, polarity is a separation of electrical charge main to a molecule or its chemical corporations having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules ought to contain polar bonds due to a distinction in electronegativity between the bonded atoms. A polar molecule with or extra polar bonds have to have a geometry that's uneven in at least one path, so that the bond dipoles do not now cancel each other.
Additional information:
The hydrogen bond is an enchantment however not a true chemical bond such as ionic or covalent bonds. It is a lot weaker. However, the hydrogen bond is a strong intermolecular bond.
Note: Polarity underlies some of bodily properties together with surface tension, solubility, and melting and boiling factors. Water is a liquid at room temperature due to H-Bond.
Complete step by step answer:
Hydrogen bonds can be intermolecular (happening among separate molecules) or intramolecular (going on among parts of the identical molecule). Depending on the character of the donor and acceptor atoms which constitute the bond, their geometry, and surroundings.

In chemistry, polarity is a separation of electrical charge main to a molecule or its chemical corporations having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules ought to contain polar bonds due to a distinction in electronegativity between the bonded atoms. A polar molecule with or extra polar bonds have to have a geometry that's uneven in at least one path, so that the bond dipoles do not now cancel each other.
Additional information:
The hydrogen bond is an enchantment however not a true chemical bond such as ionic or covalent bonds. It is a lot weaker. However, the hydrogen bond is a strong intermolecular bond.
Note: Polarity underlies some of bodily properties together with surface tension, solubility, and melting and boiling factors. Water is a liquid at room temperature due to H-Bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




