
Point out the correct decreasing order of ${\text{p}}{{\text{K}}_{\text{b}}}$values of the following amines:
${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$ ,${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}$, ${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}$and ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$
A. \[{\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\, > \,{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]
B. \[{\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,\,\,{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}\,{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}} > \,{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]
C. \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}} > {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}} > {\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,\]
D. \[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}} > {\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}} > {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}} > {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]
Answer
574.2k+ views
Hint: The basicity is defined as the tendency of a molecule to donate the electrons or hydroxide ion. AS the availability of the electrons increases, the basicity of the molecule increases. The lower the ${\text{p}}{{\text{K}}_{\text{b}}}$values higher will be the basicity.
Complete Step by step answer: In the amines, the nitrogen atom has a lone pair of electrons that can be donated so, the amines are basic.
Aliphatic amines are more basic than the aromatic amine.
Because in aromatic amine the phenyl ring works as an electron-withdrawing group which decreases the electron density on the nitrogen atom.
So, ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$ and ${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}$ are more basic than ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}$ and ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$.
In the case of aliphatic amines ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$ and ${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}$, the basicity is decided on the basis of steric hindrance and $ + {\text{I}}$ effect.
In the amine, the $ - {\text{N}}{{\text{H}}_2}$ group is an electron-withdrawing group so, the $ - {\text{N}}{{\text{H}}_2}$ group has $ + {\text{I}}$ effect. Thus, it withdraws the electron density from the alkyl chain so, as the number of alkyl groups increases the basicity should be increased.
So, according to the $ + {\text{I}}$ effect, the basicity order should be,
${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,{\text{ > }}\,{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$
The $ + {\text{I}}$ effect in ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$ and ${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}$, is as follows:
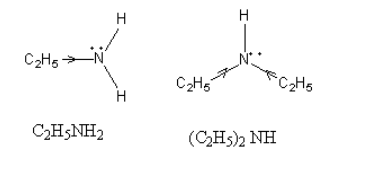
As the steric hindrance in amine increases the hydrogen ion of water which takes an electron from amine cannot approach the amine so, the basicity decreases.
So, according to the steric hindrance, the basicity order should be,
${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}} > {\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,$
The order of basicity which comes by the combined effect of $ + {\text{I}}$ and steric hindrance is as follows:
${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,{\text{ > }}\,{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$
In the case of aromatic amine${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}$ and ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$., the basicity is decided on the basis of $ + {\text{I}}$ effect of methyl group.
The $ - {\text{I}}$ effect in ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}$ and ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$, is as follows:
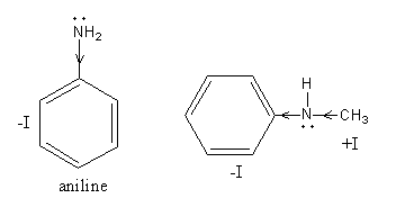
In ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}$, one methyl group is present instead of hydrogen and methyl group shows $ + {\text{I}}$effect which increases the electron density hence basicity.
So, the decreasing order of basicity is as follows:
\[{\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\, > \,{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]
${{\text{K}}_{\text{b}}}$ shows the strength of a base so, as the value of ${{\text{K}}_{\text{b}}}$ increases the basicity increases and as the${\text{p}}{{\text{K}}_{\text{b}}}$ value increases the basicity decreases.
So, a strong base will have low ${\text{p}}{{\text{K}}_{\text{b}}}$.
The decreasing order of ${\text{p}}{{\text{K}}_{\text{b}}}$values is as follows:
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}} > {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}} > {\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,\]
Therefore, option (C) is correct.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}} > {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}} > {\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,\]
Note: Basicity is directly proportional to $ + {\text{I}}$ effect and inversely proportional to the $ - {\text{I}}$ and the steric hindrance. Basicity is also directly proportional to the number of electron donating groups. As the number of electron donating groups increases the basicity increases. The basicity of aniline is minimum among all four due to $ - {\text{I}}$ of the ring.
Complete Step by step answer: In the amines, the nitrogen atom has a lone pair of electrons that can be donated so, the amines are basic.
Aliphatic amines are more basic than the aromatic amine.
Because in aromatic amine the phenyl ring works as an electron-withdrawing group which decreases the electron density on the nitrogen atom.
So, ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$ and ${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}$ are more basic than ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}$ and ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$.
In the case of aliphatic amines ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$ and ${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}$, the basicity is decided on the basis of steric hindrance and $ + {\text{I}}$ effect.
In the amine, the $ - {\text{N}}{{\text{H}}_2}$ group is an electron-withdrawing group so, the $ - {\text{N}}{{\text{H}}_2}$ group has $ + {\text{I}}$ effect. Thus, it withdraws the electron density from the alkyl chain so, as the number of alkyl groups increases the basicity should be increased.
So, according to the $ + {\text{I}}$ effect, the basicity order should be,
${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,{\text{ > }}\,{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$
The $ + {\text{I}}$ effect in ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$ and ${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}$, is as follows:

As the steric hindrance in amine increases the hydrogen ion of water which takes an electron from amine cannot approach the amine so, the basicity decreases.
So, according to the steric hindrance, the basicity order should be,
${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}} > {\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,$
The order of basicity which comes by the combined effect of $ + {\text{I}}$ and steric hindrance is as follows:
${\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,{\text{ > }}\,{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$
In the case of aromatic amine${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}$ and ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$., the basicity is decided on the basis of $ + {\text{I}}$ effect of methyl group.
The $ - {\text{I}}$ effect in ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}$ and ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}$, is as follows:

In ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}$, one methyl group is present instead of hydrogen and methyl group shows $ + {\text{I}}$effect which increases the electron density hence basicity.
So, the decreasing order of basicity is as follows:
\[{\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\, > \,{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]
${{\text{K}}_{\text{b}}}$ shows the strength of a base so, as the value of ${{\text{K}}_{\text{b}}}$ increases the basicity increases and as the${\text{p}}{{\text{K}}_{\text{b}}}$ value increases the basicity decreases.
So, a strong base will have low ${\text{p}}{{\text{K}}_{\text{b}}}$.
The decreasing order of ${\text{p}}{{\text{K}}_{\text{b}}}$values is as follows:
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}} > {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}} > {\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,\]
Therefore, option (C) is correct.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{ > }}\,{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}} > {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}} > {\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right)_2}{\text{NH}}\,\]
Note: Basicity is directly proportional to $ + {\text{I}}$ effect and inversely proportional to the $ - {\text{I}}$ and the steric hindrance. Basicity is also directly proportional to the number of electron donating groups. As the number of electron donating groups increases the basicity increases. The basicity of aniline is minimum among all four due to $ - {\text{I}}$ of the ring.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




