
Plot P versus $\dfrac{1}{V}$ at different temperatures.
Answer
561.6k+ views
Hint: We know Boyle’s Law:
According to Boyle's law, as the pressure decreases the volume increases thus, the bubbles at the bottom of a glass of a soft drink get larger when it rises to the surface because when the bubbles rise, the pressure inside the bubble starts to get reduced and the volume (size) of the bubble increases.
At constant temperature, the pressure and the volume of the gas are inversely related.
${\text{PV = K}}$
Where P is the pressure of the gas at a constant temperature.
V is the volume of the gas at a constant temperature.
k is the constant.
Complete step by step answer:
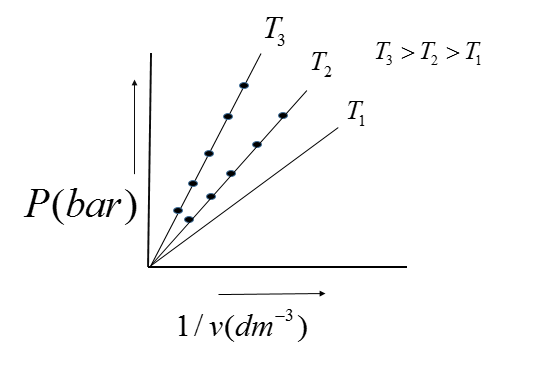
The graph is a straight line through the origin, verifying that the pressure is proportional to $\dfrac{1}{V}$ verifying Boyle's Law. Also, all values of pressure and volume are the same.
$P \propto$ $\dfrac{1}{V}$
$p = k\dfrac{1}{V}$
Therefore, when different values of pressure are plotted against the reciprocals of the respective $\dfrac{1}{V}$ volume. This graph justifies Boyle's law because the straight line is obtained which passes through the origin.
So, the correct answer is Option D.
Note: We need to know that the Boyles' law explains the reason why the bubble at the bottom of a glass of a soft drink gets larger when it rises to the surface and why the bag of potato chips on a plane ride appeared to be inflated when taken out, We need to know that the bag of potato chips on a plane ride appears to be inflated when taken out because according to Boyle's law whenever the pressure of a gaseous system decreases its volume increases at a constant temperature. At the higher height, the pressure is reduced so the gas inside the bag becomes inflated.
According to Boyle's law, as the pressure decreases the volume increases thus, the bubbles at the bottom of a glass of a soft drink get larger when it rises to the surface because when the bubbles rise, the pressure inside the bubble starts to get reduced and the volume (size) of the bubble increases.
At constant temperature, the pressure and the volume of the gas are inversely related.
${\text{PV = K}}$
Where P is the pressure of the gas at a constant temperature.
V is the volume of the gas at a constant temperature.
k is the constant.
Complete step by step answer:

The graph is a straight line through the origin, verifying that the pressure is proportional to $\dfrac{1}{V}$ verifying Boyle's Law. Also, all values of pressure and volume are the same.
$P \propto$ $\dfrac{1}{V}$
$p = k\dfrac{1}{V}$
Therefore, when different values of pressure are plotted against the reciprocals of the respective $\dfrac{1}{V}$ volume. This graph justifies Boyle's law because the straight line is obtained which passes through the origin.
So, the correct answer is Option D.
Note: We need to know that the Boyles' law explains the reason why the bubble at the bottom of a glass of a soft drink gets larger when it rises to the surface and why the bag of potato chips on a plane ride appeared to be inflated when taken out, We need to know that the bag of potato chips on a plane ride appears to be inflated when taken out because according to Boyle's law whenever the pressure of a gaseous system decreases its volume increases at a constant temperature. At the higher height, the pressure is reduced so the gas inside the bag becomes inflated.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Class 11 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




