
Please explain the $ + E$ and $ - E$ electromeric effect with examples.
Answer
496.5k+ views
Hint: The instantaneous formation of a dipole in an organic compound because of complete transfer of shared pi electron pairs to one of the atoms having double bond under the influence of an attacking reagent is referred to as an electromeric effect. It is a temporary effect that means the molecule will return back to its original state when the attacking reagent is removed from the system.
Complete answer:
Electromeric effect can be classified into two categories i.e., $ + E$ and $ - E$ electromeric effect. The classification is based on the criteria in which direction in which the electron pair is transferred which is explained as follows:
$ + E$ electromeric effect:
This effect is observed when the electron pair of the pi bond is transferred towards the attacking reagent. It is generally observed when an attacking reagent is an electrophile i.e., electron deficient species and will attract the pi electrons towards it. A common example for the $ + E$ effect is the addition of an acid to alkenes.
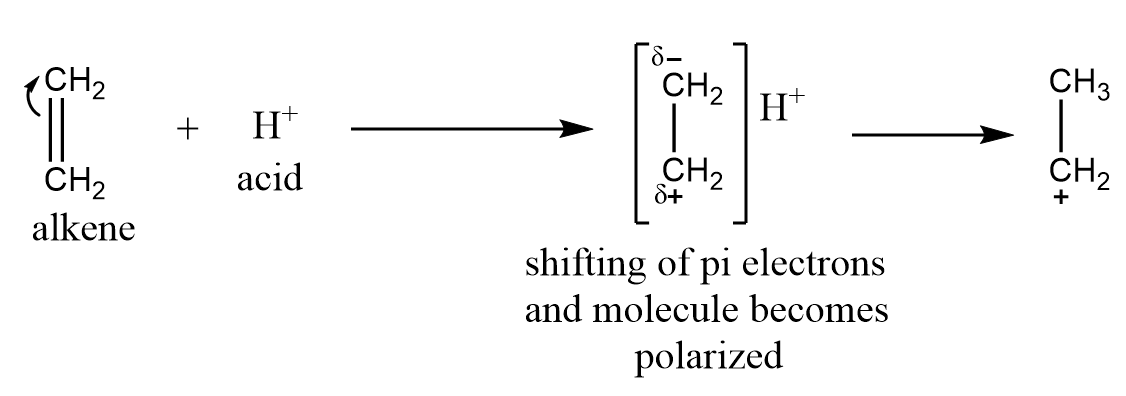
$ - E$ electromeric effect:
This effect occurs when the electron pair of the pi bond is shifted away from the attacking reagent. In this case, the attacking reagent is a nucleophile i.e., electron rich species and thus, forms a bond with the positively charged atom in the molecule instead of the atom to which the pi electrons are transferred. A common example of the $ - E$ effect is addition of a nucleophile to a carbonyl compound.
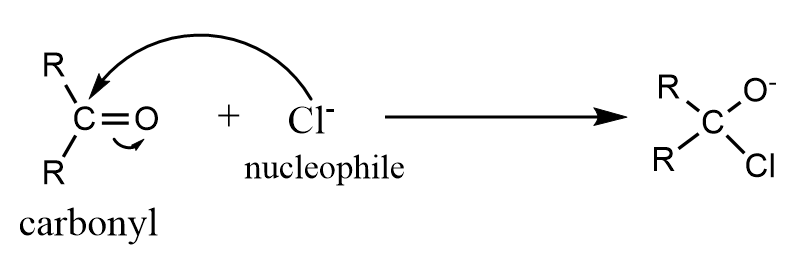
Note:
It is important to note that unlike the inductive effect, the electromeric effect is not a permanent effect which only arises when the compound is subjected to an attacking reagent and this effect is observed only in those organic compounds in which multiple bonds are present.
Complete answer:
Electromeric effect can be classified into two categories i.e., $ + E$ and $ - E$ electromeric effect. The classification is based on the criteria in which direction in which the electron pair is transferred which is explained as follows:
$ + E$ electromeric effect:
This effect is observed when the electron pair of the pi bond is transferred towards the attacking reagent. It is generally observed when an attacking reagent is an electrophile i.e., electron deficient species and will attract the pi electrons towards it. A common example for the $ + E$ effect is the addition of an acid to alkenes.

$ - E$ electromeric effect:
This effect occurs when the electron pair of the pi bond is shifted away from the attacking reagent. In this case, the attacking reagent is a nucleophile i.e., electron rich species and thus, forms a bond with the positively charged atom in the molecule instead of the atom to which the pi electrons are transferred. A common example of the $ - E$ effect is addition of a nucleophile to a carbonyl compound.
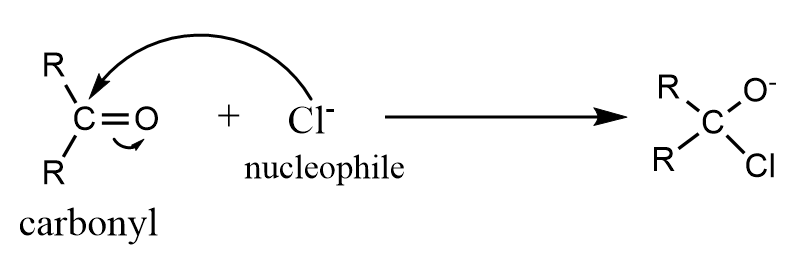
Note:
It is important to note that unlike the inductive effect, the electromeric effect is not a permanent effect which only arises when the compound is subjected to an attacking reagent and this effect is observed only in those organic compounds in which multiple bonds are present.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




