
How many planes are present in $4{d_{{z^2}}}$?
a.$2$
b.$1$
c.$Zero$
d.$3$
Answer
478.5k+ views
Hint: The planes where there is zero probability region to find the electrons are known as nodal planes. The number of planes is equal to $l$ which is an azimuthal quantum number. We can find the coordinates of these planes by solving the Schrodinger wave equation which is for atoms or molecules to find the shapes of the atomic and molecular orbital.
Complete answer:
Nodal planes are areas that are around the atomic nuclei where the chances of finding electrons are zero. The shape of an atomic orbital is given by the density of the electron cloud, which indicates the chances of finding electrons in the area surrounding the atom or molecule. The denser the cloud would be, the higher we have the chances of finding an electron. Nodal planes lack electron cloud density which implies that the chances of finding electrons in these planes are probably zero. The nodal planes tell us about the wave nature of the electrons because nodes are the point of zero amplitude along with the wave. If there is no amplitude of vibration that means there is no electron density present.
In the question, the structure of $4{d_{{z^2}}}$ is:
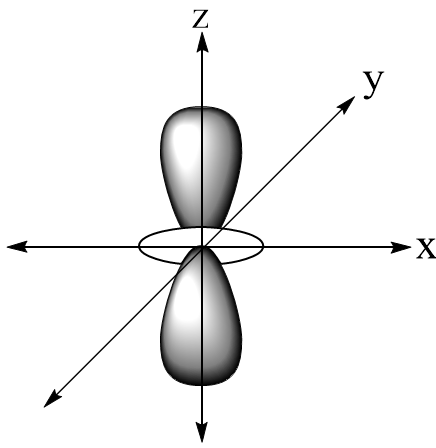
Here we can see that there is the electron density in each plane. A nodal plane is a plane that has zero electron density thus $4{d_{{z^2}}}$ has zero nodal planes.
Thus, option $\left( c \right)$ is correct.
Note:
The point which has zero probability of finding the electron is termed a nodal point. There are two types of nodes:
Radial nodes
Angular nodes
Complete answer:
Nodal planes are areas that are around the atomic nuclei where the chances of finding electrons are zero. The shape of an atomic orbital is given by the density of the electron cloud, which indicates the chances of finding electrons in the area surrounding the atom or molecule. The denser the cloud would be, the higher we have the chances of finding an electron. Nodal planes lack electron cloud density which implies that the chances of finding electrons in these planes are probably zero. The nodal planes tell us about the wave nature of the electrons because nodes are the point of zero amplitude along with the wave. If there is no amplitude of vibration that means there is no electron density present.
In the question, the structure of $4{d_{{z^2}}}$ is:

Here we can see that there is the electron density in each plane. A nodal plane is a plane that has zero electron density thus $4{d_{{z^2}}}$ has zero nodal planes.
Thus, option $\left( c \right)$ is correct.
Note:
The point which has zero probability of finding the electron is termed a nodal point. There are two types of nodes:
Radial nodes
Angular nodes
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




