
How many $\pi $-electrons are present in the ferrocene?
Answer
570k+ views
Hint: Ferrocene can be stated as an organometallic compound consisting of transition elements. In order to find ferrocene we have to draw the structure of ferrocene, which will make it easier for finding the $\pi $-electrons present in it.
Complete Step by step answer: First of all let us know what organometallic compounds are. They are compounds containing at least one bond between a metal and a carbon atom of an organic compound namely like alkaline earth metals or transition metals.
Coming to ferrocene, it is an organometallic compound with a bond between iron atoms. Here the central atom is iron and they are bounded by two cyclopentadienyl rings. The molecular formula of ferrocene is $Fe{\left( {{C_5}{H_5}} \right)_2}$
Now, let us look into the $\pi $-bond, it is formed by the sideways or lateral overlapping of p-orbital. In $\pi $-bond formation, the extent of overlapping is very small, hence $\pi $-bond is a weak bond. $\pi $-bond cannot exist independently. Firstly, ${{\sigma }}$-bond is formed and then only $\pi $-bond formation may take place. In multiple bonds, one bond is -bond and the rest of the bonds are $\pi $ -bonds.
Now, let us look at the structure of ferrocene
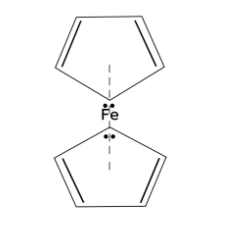
Here we can see two rings of cyclopentadienyl which have two double bonds and a pair of electrons. Since it has double bonds, we can say it has one ${{\sigma }}$-bond and one $\pi $-bond. The $\pi $-bond will have 2 electrons, hence in one ring there is a total of 4 electrons due to double bonds and one extra pair of electrons makes the total as six. In the ferrocene there are two rings therefore the total number of $\pi $-electrons becomes 12.
Hence there is 12 $\pi $-electrons available in the ferrocene.
Note: Ferrocene has a lot of industrial application; many industrial processes really depend on organometallic compounds which include alkene derived polymers. Also, several semiconductors are also derived from the organometallic compounds.
Complete Step by step answer: First of all let us know what organometallic compounds are. They are compounds containing at least one bond between a metal and a carbon atom of an organic compound namely like alkaline earth metals or transition metals.
Coming to ferrocene, it is an organometallic compound with a bond between iron atoms. Here the central atom is iron and they are bounded by two cyclopentadienyl rings. The molecular formula of ferrocene is $Fe{\left( {{C_5}{H_5}} \right)_2}$
Now, let us look into the $\pi $-bond, it is formed by the sideways or lateral overlapping of p-orbital. In $\pi $-bond formation, the extent of overlapping is very small, hence $\pi $-bond is a weak bond. $\pi $-bond cannot exist independently. Firstly, ${{\sigma }}$-bond is formed and then only $\pi $-bond formation may take place. In multiple bonds, one bond is -bond and the rest of the bonds are $\pi $ -bonds.
Now, let us look at the structure of ferrocene

Here we can see two rings of cyclopentadienyl which have two double bonds and a pair of electrons. Since it has double bonds, we can say it has one ${{\sigma }}$-bond and one $\pi $-bond. The $\pi $-bond will have 2 electrons, hence in one ring there is a total of 4 electrons due to double bonds and one extra pair of electrons makes the total as six. In the ferrocene there are two rings therefore the total number of $\pi $-electrons becomes 12.
Hence there is 12 $\pi $-electrons available in the ferrocene.
Note: Ferrocene has a lot of industrial application; many industrial processes really depend on organometallic compounds which include alkene derived polymers. Also, several semiconductors are also derived from the organometallic compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




