
How many pi bonds in $ {{C}_{2}}{{H}_{6}} $ ? Write it’s structure.
Answer
551.1k+ views
Hint: We are already provided with the compound. The given compound is ethane. This question could be simply solved from the concepts of alkane, alkene and alkyne. It is a part of organic chemistry. Pi bonds are present in alkenes and alkynes.
Complete step by step answer
We already know that the given compound is ethane.
It is an alkane with a general formula of $ {{C}_{n}}{{H}_{2n+2}} $ .
Alkanes do not have double bonds.
Thus, there are no pi bonds in $ {{C}_{2}}{{H}_{6}} $
Its structure is:
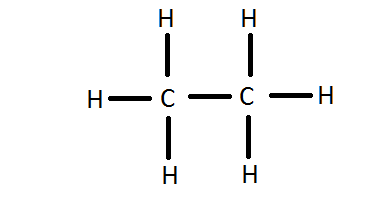
Additional Information
We should know that two of the strongest forms of chemical bonds are the ionic and the covalent bonds. Chemical bonds form between two atoms, each with its own electron environment. Sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Covalent bonds are formed by the overlapping of atomic orbitals. Sigma bonds are a result of the head-to-head overlapping of atomic orbitals whereas pi bonds are formed by the lateral overlap of two atomic orbitals. Usually, all bonds between atoms in most organic compounds contain one sigma bond each. If it is a single bond, it contains only a sigma bond. Double and Triple bonds, however, contain sigma and pi bonds. Double bonds have one each, and triple bonds have one sigma bond and two pi bonds.
Note
We should also know that a pi bond is a weaker chemical covalent bond than a sigma bond (since pi bonds have a smaller overlap between the orbitals), but when it is put with a sigma bond it creates a much stronger hold between the atoms, thus double and triple bonds are stronger than single bonds. Also, the pi bonds are present only in alkenes and alkynes.
Complete step by step answer
We already know that the given compound is ethane.
It is an alkane with a general formula of $ {{C}_{n}}{{H}_{2n+2}} $ .
Alkanes do not have double bonds.
Thus, there are no pi bonds in $ {{C}_{2}}{{H}_{6}} $
Its structure is:

Additional Information
We should know that two of the strongest forms of chemical bonds are the ionic and the covalent bonds. Chemical bonds form between two atoms, each with its own electron environment. Sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Covalent bonds are formed by the overlapping of atomic orbitals. Sigma bonds are a result of the head-to-head overlapping of atomic orbitals whereas pi bonds are formed by the lateral overlap of two atomic orbitals. Usually, all bonds between atoms in most organic compounds contain one sigma bond each. If it is a single bond, it contains only a sigma bond. Double and Triple bonds, however, contain sigma and pi bonds. Double bonds have one each, and triple bonds have one sigma bond and two pi bonds.
Note
We should also know that a pi bond is a weaker chemical covalent bond than a sigma bond (since pi bonds have a smaller overlap between the orbitals), but when it is put with a sigma bond it creates a much stronger hold between the atoms, thus double and triple bonds are stronger than single bonds. Also, the pi bonds are present only in alkenes and alkynes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




