
What physical property is identical for the isomer of ${C_5}{H_{12}}$ ?
Answer
521.4k+ views
Hint: We have to know that isomerism is the marvel where more than one mixtures have similar synthetic recipes yet extraordinary substance structures. Substance intensifies that have indistinguishable synthetic formulae however contrast in properties and the game plan of particles in the atom are called isomers. Accordingly, the mixtures that show isomerism are known as isomers.
Complete answer:
We have to see that there are two essential sorts of isomerism, which can be additionally arranged into various subtypes. These essential kinds are structural Isomerism and Stereoisomerism.
When underlying the structural isomerism is normally alluded to as protected isomerism. The practical gatherings and the particles in the atoms of these isomers are connected in an unexpected way. Distinctive primary isomers are relegated diverse IUPAC names since they might possibly contain a similar useful gathering. It is otherwise called skeletal isomerism. The segments of these isomers show diversely fanned designs. Regularly, chain isomers contrast in the spreading of carbon. An illustration of chain isomerism can be seen in the compound ${C_5}{H_{12}}$ , as shown underneath. There are three types, namely pentane, isopentane and neopentane.
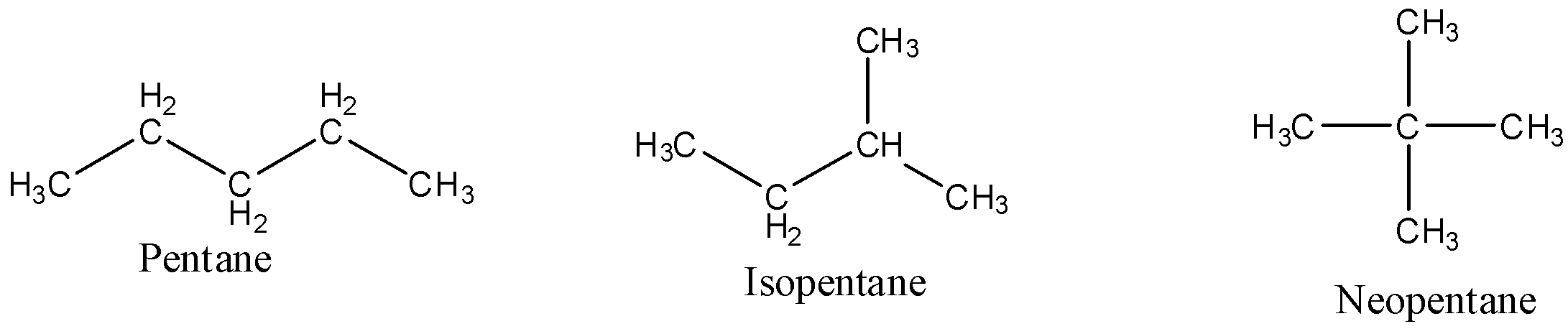
When the molar mass is indistinguishable for the isomers of ${C_5}{H_{12}}$ .
The sub-atomic mass is one property that essentially stays consistent for every one of the isomeric structures.
Note:
We have to see that the hydrocarbons with at least four carbon particles have isomers. The more carbon particles a hydrocarbon has, the more noteworthy the quantity of isomers. Isomers are various mixtures with various properties, like diverse bubbling and softening focuses.
Complete answer:
We have to see that there are two essential sorts of isomerism, which can be additionally arranged into various subtypes. These essential kinds are structural Isomerism and Stereoisomerism.
When underlying the structural isomerism is normally alluded to as protected isomerism. The practical gatherings and the particles in the atoms of these isomers are connected in an unexpected way. Distinctive primary isomers are relegated diverse IUPAC names since they might possibly contain a similar useful gathering. It is otherwise called skeletal isomerism. The segments of these isomers show diversely fanned designs. Regularly, chain isomers contrast in the spreading of carbon. An illustration of chain isomerism can be seen in the compound ${C_5}{H_{12}}$ , as shown underneath. There are three types, namely pentane, isopentane and neopentane.

When the molar mass is indistinguishable for the isomers of ${C_5}{H_{12}}$ .
The sub-atomic mass is one property that essentially stays consistent for every one of the isomeric structures.
Note:
We have to see that the hydrocarbons with at least four carbon particles have isomers. The more carbon particles a hydrocarbon has, the more noteworthy the quantity of isomers. Isomers are various mixtures with various properties, like diverse bubbling and softening focuses.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




