
Photographic paper is developed with alkaline hydroquinone.
Select the correct answer.
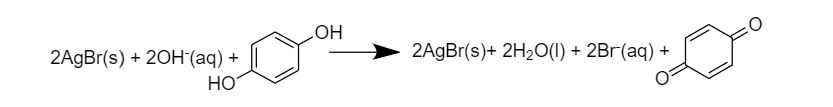
A) Hydroquinone is the oxidant
B) $A{{g}^{+}}$is the oxidant
C) $B{{r}^{-}}$is the oxidant
D) $A{{g}^{+}}$is the reductant

Answer
533.1k+ views
Hint: Before solving this question, we should first know about the oxidants and reductants and then we can see which compound is getting oxidized and which of them is getting reduced. Oxidants are the compounds that accept the electron and Reductants are the compounds that donate the electron.
Complete answer:
Hydroquinone comes under the category of phenol is an aromatic organic compound that has two hydroxyl groups joined/bonded to the benzene ring occupying the para position. It is a derivative of benzene which is a granular solid and is white.
The production of hydroquinone is can happen in two ways-
The reaction mechanism of this process is similar to the cumene process. In this, the alkylation of benzene happens with the propene to give diisopropyl benzene, then it reacts with air to provide hydroperoxide and reposition in acid to give acetone and hydroquinone.
Another method is the hydroxylation of phenol with the help of a catalyst. The reaction happens between phenol and hydrogen peroxide and it gives a mixture of catechol and hydroquinone.
${{C}_{6}}{{H}_{5}}OH\,+\,{{H}_{2}}{{O}_{2\,}}\,\to \,{{C}_{6}}{{H}_{4}}{{(OH)}_{2}}\,+\,{{H}_{2}}O$
In the reaction
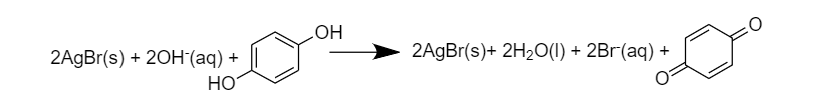
$A{{g}^{+}}$is getting reduced to $Ag$-
$2AgBr\,\to \,Ag\,+\,B{{r}^{-}}$
Therefore, $A{{g}^{+}}$is the oxidant
Whereas Hydroquinone is getting oxidized to quinone-
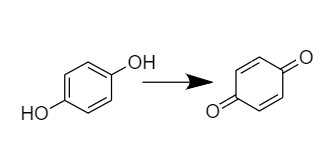
Therefore, $A{{g}^{+}}$ is the oxidant
So option (B) $A{{g}^{+}}$ is the oxidant is correct
So, the correct answer is “Option B”.
Note:
There are various applications of Hydroquinone- They are used as photographic developers for film and paper. Silver halides are reduced to silver with the help of hydroquinone, They are also used as a polymerization inhibitor. It is also used to lighten the dark patches on the skin.
Complete answer:
Hydroquinone comes under the category of phenol is an aromatic organic compound that has two hydroxyl groups joined/bonded to the benzene ring occupying the para position. It is a derivative of benzene which is a granular solid and is white.
The production of hydroquinone is can happen in two ways-
The reaction mechanism of this process is similar to the cumene process. In this, the alkylation of benzene happens with the propene to give diisopropyl benzene, then it reacts with air to provide hydroperoxide and reposition in acid to give acetone and hydroquinone.
Another method is the hydroxylation of phenol with the help of a catalyst. The reaction happens between phenol and hydrogen peroxide and it gives a mixture of catechol and hydroquinone.
${{C}_{6}}{{H}_{5}}OH\,+\,{{H}_{2}}{{O}_{2\,}}\,\to \,{{C}_{6}}{{H}_{4}}{{(OH)}_{2}}\,+\,{{H}_{2}}O$
In the reaction

$A{{g}^{+}}$is getting reduced to $Ag$-
$2AgBr\,\to \,Ag\,+\,B{{r}^{-}}$
Therefore, $A{{g}^{+}}$is the oxidant
Whereas Hydroquinone is getting oxidized to quinone-

Therefore, $A{{g}^{+}}$ is the oxidant
So option (B) $A{{g}^{+}}$ is the oxidant is correct
So, the correct answer is “Option B”.
Note:
There are various applications of Hydroquinone- They are used as photographic developers for film and paper. Silver halides are reduced to silver with the help of hydroquinone, They are also used as a polymerization inhibitor. It is also used to lighten the dark patches on the skin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




