
Phenyl isocyanide is prepared from aniline by:
(a)- Rosenmund’s reaction
(b)- Kolbe’s reaction
(c)- Reimer-Tiemann reaction
(d)- Fittig reaction
(e)- Carbylamine reaction.
Answer
594.9k+ views
Hint: The catalyst used is chloroform and an alcoholic solution of potassium hydroxide. This reaction is a type of test reaction that is used to distinguish between primary amines from secondary and tertiary amines.
Complete step by step solution:
Let us study all the options one by one:
(a)- Rosenmund reaction.
Acid chlorides are easily reduced to the corresponding aldehydes by passing hydrogen gas through boiling xylene solution of the acid chloride in the presence of a Pd catalyst supported over\[BaS{{O}_{4}}\].
\[RCOCl+{{H}_{2}}\to RCOH+HCl\]
(b)- Kolbe’s reaction.
Sodium phenoxide when heated with carbon dioxide at 400 K under a pressure of 4-7 atmosphere followed by acidification gives 2-hydroxybenzoic acid (salicylic acid) as the main product.
(c)- Reimer-Tiemann reaction.
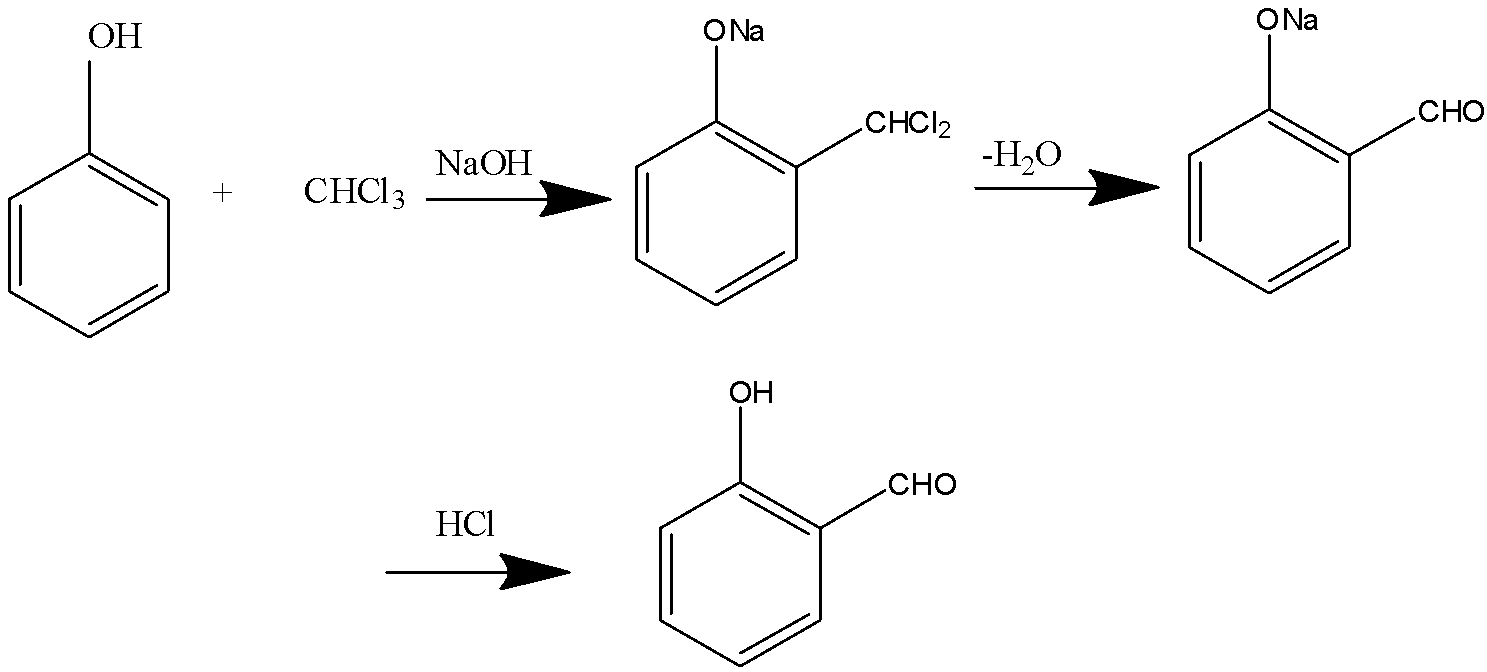
Treatment of phenol with chloroform in the presence of aqueous or potassium hydroxide at 340K followed by hydrolysis of the resulting product gives 2-hydroxybenzaldehyde (salicylaldehyde) as the major product.
(d)- Fittig reaction.
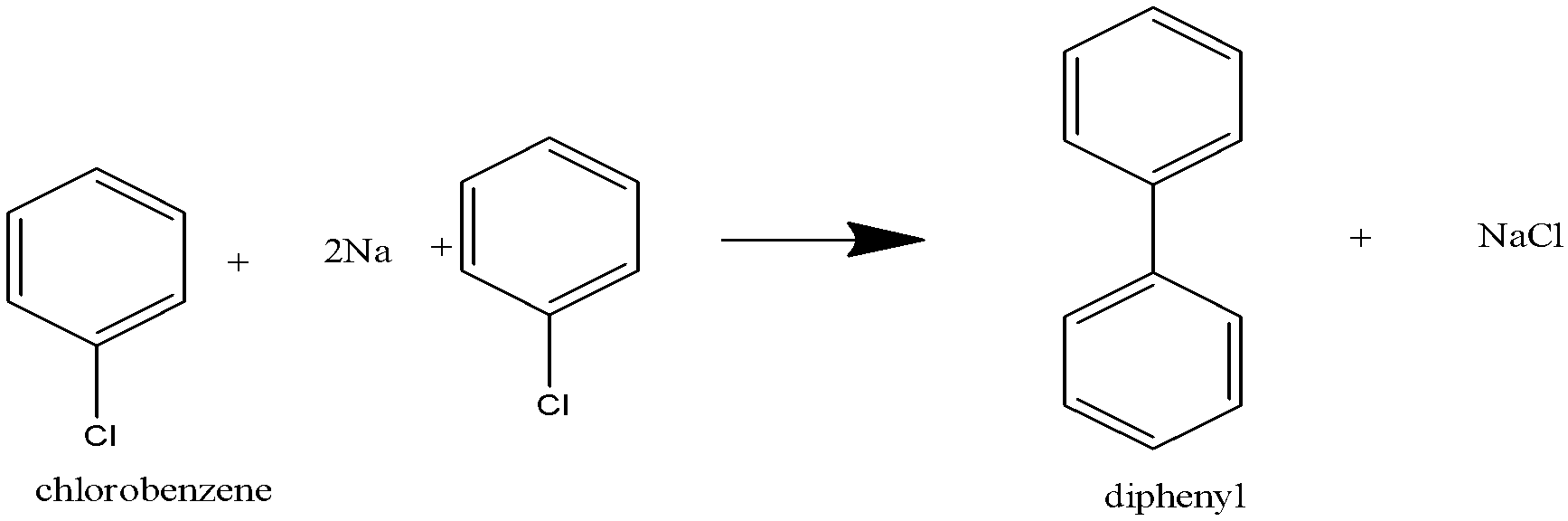
When only haloarenes are treated with sodium, diaryls are produced.
(e)- Carbylamine reaction.
Both aliphatic and aromatic primary amines when warmed with chloroform and an alcoholic solution of KOH produce isocyanides or carbylamines.
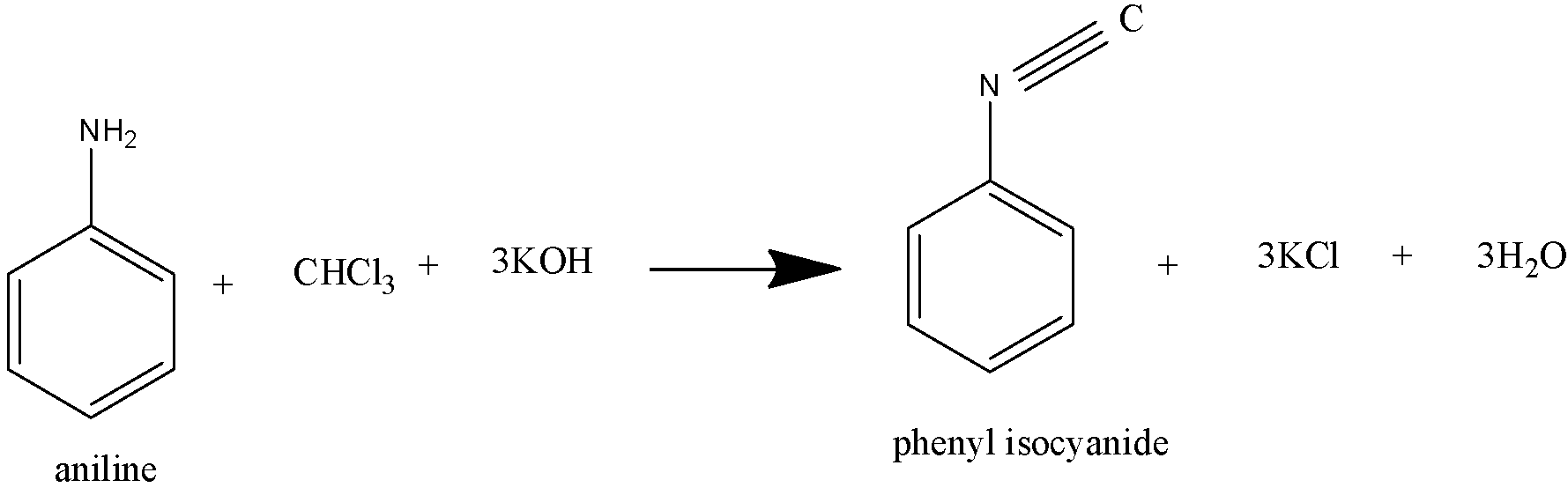
So, from the above observation, we can see that aniline is converted into phenyl isocyanide in the carbylamine reaction.
So, the correct answer is “Option E”.
Note: Don’t get confused between reamer-teimann and carbylamines reaction because both of them have chloroform as one of the reactants. In contrast, secondary and tertiary amines do not give the carbylamine reaction.
Complete step by step solution:
Let us study all the options one by one:
(a)- Rosenmund reaction.
Acid chlorides are easily reduced to the corresponding aldehydes by passing hydrogen gas through boiling xylene solution of the acid chloride in the presence of a Pd catalyst supported over\[BaS{{O}_{4}}\].
\[RCOCl+{{H}_{2}}\to RCOH+HCl\]
(b)- Kolbe’s reaction.
Sodium phenoxide when heated with carbon dioxide at 400 K under a pressure of 4-7 atmosphere followed by acidification gives 2-hydroxybenzoic acid (salicylic acid) as the main product.
(c)- Reimer-Tiemann reaction.
Treatment of phenol with chloroform in the presence of aqueous or potassium hydroxide at 340K followed by hydrolysis of the resulting product gives 2-hydroxybenzaldehyde (salicylaldehyde) as the major product.

(d)- Fittig reaction.
When only haloarenes are treated with sodium, diaryls are produced.

(e)- Carbylamine reaction.
Both aliphatic and aromatic primary amines when warmed with chloroform and an alcoholic solution of KOH produce isocyanides or carbylamines.

So, from the above observation, we can see that aniline is converted into phenyl isocyanide in the carbylamine reaction.
So, the correct answer is “Option E”.
Note: Don’t get confused between reamer-teimann and carbylamines reaction because both of them have chloroform as one of the reactants. In contrast, secondary and tertiary amines do not give the carbylamine reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




