
How is phenol prepared from?
(i) Isopropyl benzene.
(ii) Benzene sulphonic acid.
Answer
579.6k+ views
Hint: Phenol is aromatic hydroxy alcohol in which a hydroxyl group (OH-) is directly attached with the benzene ring. Phenol is an acidic compound because of the resonance stabilization of phenoxide ions. Phenol is industrially prepared by ‘Dow Process’.
Complete answer: Phenol is prepared by different methods, such as from-
(i) Isopropyl benzene –IUPAC name of isopropyl benzene is 1-methylethyl benzene, which is commonly known as cumene. This method is the recent and best method to form phenol from cumene. Cumene on oxidation gives cumene hydroperoxide which, on hydrolysis, gives phenol and acetone as a by-product. This reaction is completed in two steps –
(I) cumene is oxidised in presence of air at 400K in the presence of metal catalyst to form cumene hydroperoxide.
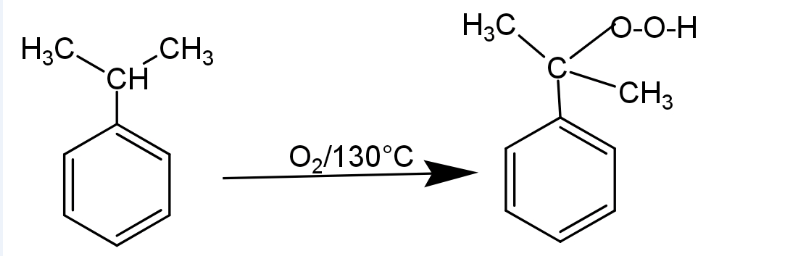
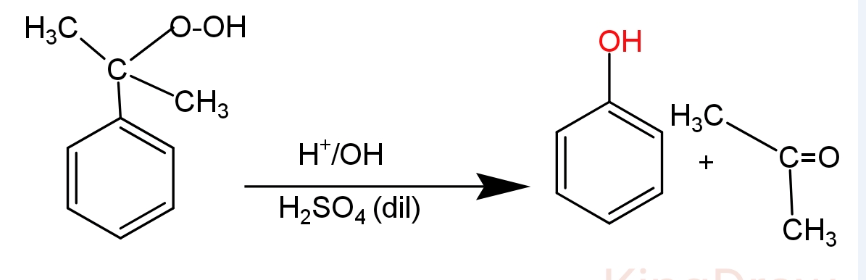
(II) In the second step cumene hydroperoxide is treated with dilute sulphuric acid at 350K. It causes hydrolysis and forms phenol and acetone.
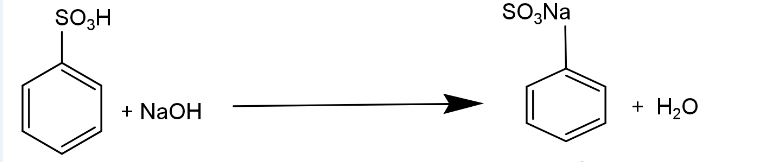
(ii) Benzene sulphonic acid – Benzene sulphonic acid is the organo-sulphur compound of benzene. Sodium salt of benzene sulphonic acid is fused with caustic soda to form sodium phenoxide, which produces phenol by acid decomposition.

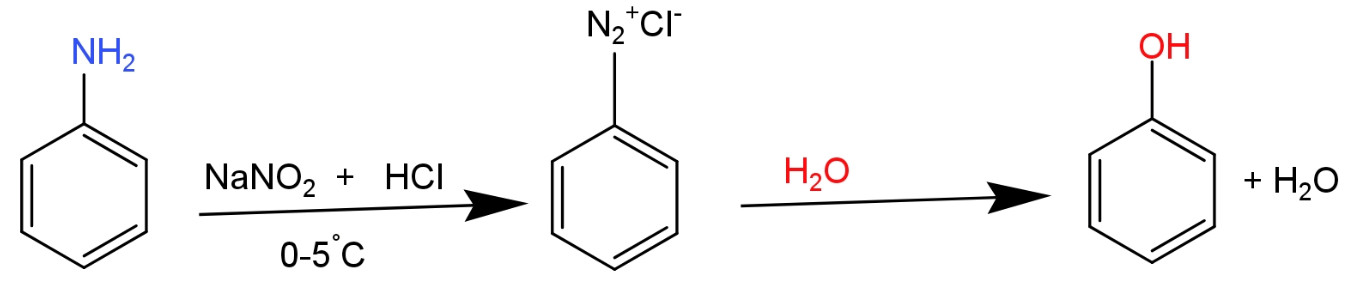
Note: phenol is also prepared by aniline. In this method firstly aniline is converted into diazonium salt by treating with sodium nitrite and hydrochloric acid at low temperature. This reaction is known as diazotization. An aqueous solution of benzenediazonium salt gives phenol after heating with sulphuric acid.
Complete answer: Phenol is prepared by different methods, such as from-
(i) Isopropyl benzene –IUPAC name of isopropyl benzene is 1-methylethyl benzene, which is commonly known as cumene. This method is the recent and best method to form phenol from cumene. Cumene on oxidation gives cumene hydroperoxide which, on hydrolysis, gives phenol and acetone as a by-product. This reaction is completed in two steps –
(I) cumene is oxidised in presence of air at 400K in the presence of metal catalyst to form cumene hydroperoxide.

(II) In the second step cumene hydroperoxide is treated with dilute sulphuric acid at 350K. It causes hydrolysis and forms phenol and acetone.

(ii) Benzene sulphonic acid – Benzene sulphonic acid is the organo-sulphur compound of benzene. Sodium salt of benzene sulphonic acid is fused with caustic soda to form sodium phenoxide, which produces phenol by acid decomposition.


Note: phenol is also prepared by aniline. In this method firstly aniline is converted into diazonium salt by treating with sodium nitrite and hydrochloric acid at low temperature. This reaction is known as diazotization. An aqueous solution of benzenediazonium salt gives phenol after heating with sulphuric acid.

Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

How is the angle of emergence e related to the angle class 12 physics CBSE

Differentiate between lanthanoids and actinoids class 12 chemistry CBSE

Derive Lens Makers formula for a convex lens class 12 physics CBSE

a Draw Labelled diagram of Standard Hydrogen Electrode class 12 chemistry CBSE




