
Part of the periodic table showing p-block is depicted below. What are the elements shown in zig-zag boxes called? What is the nature of the elements outside this boundary on the right side of the table?
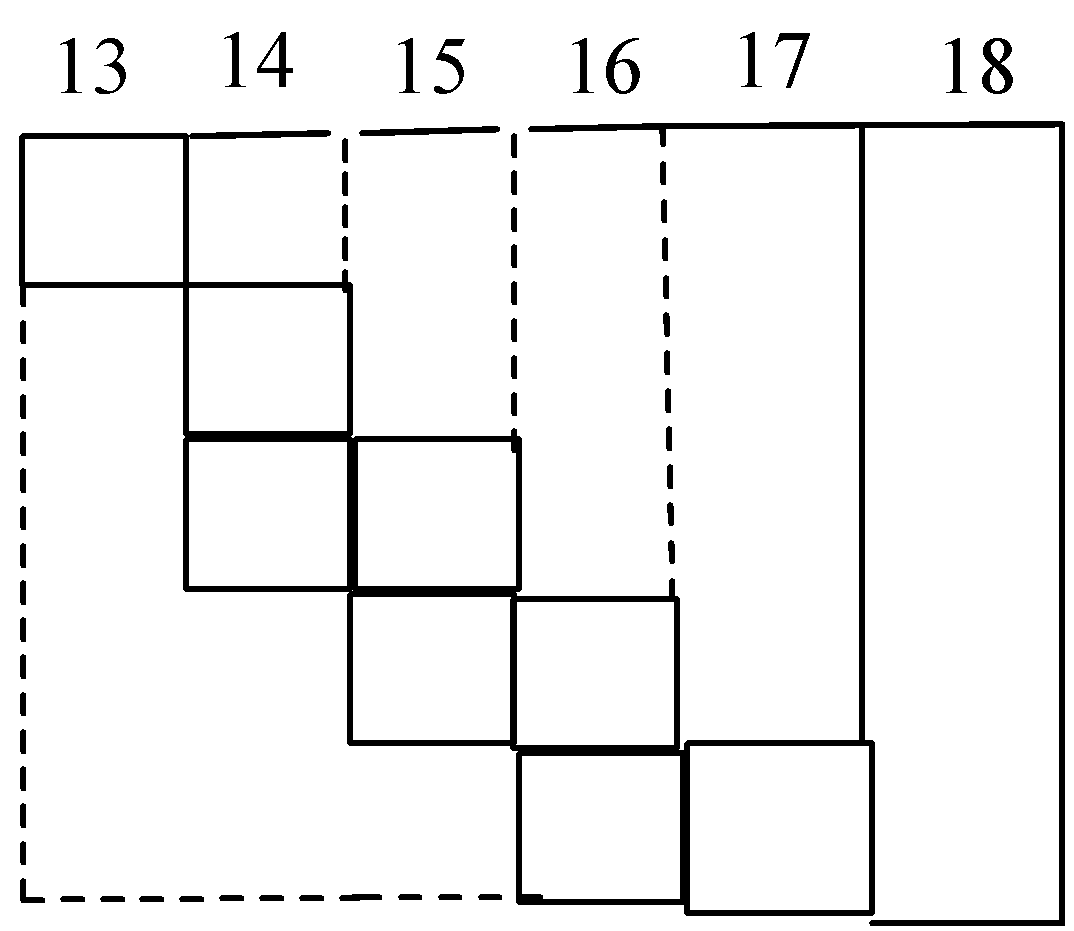
A. Transition elements, metalloids
B.Metalloids, non-metals
C.Metals, non-metals
D.Non-metals, noble gases

Answer
558.6k+ views
Hint:We know that the periodic table is divided into four parts namely s-block, p-block, d-block and f-block. The s-block consists of metals, d-block consists of transition metals and f-block consists of inner transition metals.
Complete step by step answer:
Let’s discuss the p-block of the periodic table in detail. Group number 13 to 18 is called p-block. This block consists of non-metals and metalloids. Metalloids are the elements that possess properties of both metals and nonmetals, such as boron, silicon etc. The elements present in zig-zag boxes are called metalloids. And the remaining elements of the periodic table are non-metals. Non metals are the elements that can accept electrons to acquire stable electronic configuration. Complete table is,
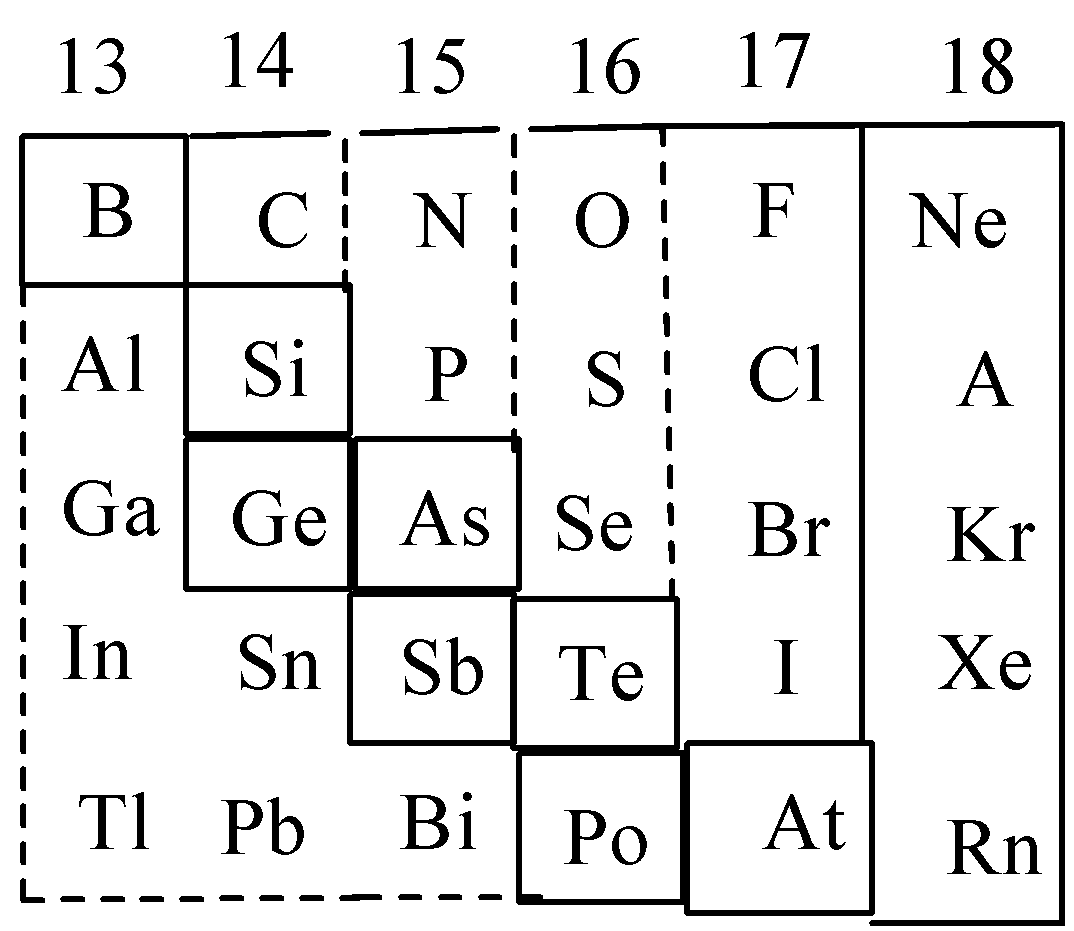
Therefore, the correct choice is option B.
Additional Information:
Let’s discuss s block, d-block and f-block of the periodic table in detail.
The s-block includes those elements in which the last electron is filled in the valence s-subshell of the outermost energy shell. Since s-subshell can have a maximum of two electrons only, therefore only two groups (1 and 2) constitute this block. The group 1 elements are called as alkali metals and group 2 elements are known as alkaline earth metals.
The d block of the periodic table includes those elements in which the last electron enters the d-sub-shell of the last but one (penultimate) energy level. For d-block elements, the general electronic configuration is $\left( {n - 1} \right){d^{1 - 10}}n{s^{1 - 2}}$.
In the f-block of the periodic table, the filling of the last electron takes place in the (n-2)f subshell. At the same time, (n-1)d sub shell may contain zero or one electron while the subshell has two electrons. For f-block elements, the general electronic configuration is$\left( {n - 2} \right){f^{1 - 14}}\left( {n - 1} \right){d^{0 - 1}}n{s^2}$.
Note:
It is to be noted that in the p-block, group 18 is termed as noble gases, group 17 is termed as halogens and group 16 is termed as chalcogens. The elements of p block and s-block are collectively termed as representative elements.
Complete step by step answer:
Let’s discuss the p-block of the periodic table in detail. Group number 13 to 18 is called p-block. This block consists of non-metals and metalloids. Metalloids are the elements that possess properties of both metals and nonmetals, such as boron, silicon etc. The elements present in zig-zag boxes are called metalloids. And the remaining elements of the periodic table are non-metals. Non metals are the elements that can accept electrons to acquire stable electronic configuration. Complete table is,

Therefore, the correct choice is option B.
Additional Information:
Let’s discuss s block, d-block and f-block of the periodic table in detail.
The s-block includes those elements in which the last electron is filled in the valence s-subshell of the outermost energy shell. Since s-subshell can have a maximum of two electrons only, therefore only two groups (1 and 2) constitute this block. The group 1 elements are called as alkali metals and group 2 elements are known as alkaline earth metals.
The d block of the periodic table includes those elements in which the last electron enters the d-sub-shell of the last but one (penultimate) energy level. For d-block elements, the general electronic configuration is $\left( {n - 1} \right){d^{1 - 10}}n{s^{1 - 2}}$.
In the f-block of the periodic table, the filling of the last electron takes place in the (n-2)f subshell. At the same time, (n-1)d sub shell may contain zero or one electron while the subshell has two electrons. For f-block elements, the general electronic configuration is$\left( {n - 2} \right){f^{1 - 14}}\left( {n - 1} \right){d^{0 - 1}}n{s^2}$.
Note:
It is to be noted that in the p-block, group 18 is termed as noble gases, group 17 is termed as halogens and group 16 is termed as chalcogens. The elements of p block and s-block are collectively termed as representative elements.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




