
Packing of $N{{a}^{+}}$ and $C{{l}^{-}}$ ions in sodium chloride is depicted by the given figure, choose the correct option regarding formula mass of sodium chloride.
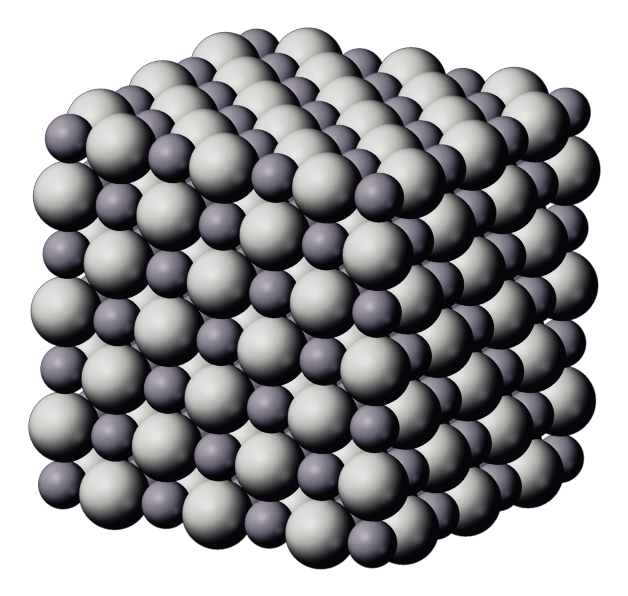
A. In the solid state, sodium chloride does not exist as a single entity.
B. formula mass of NaCl is 68.0 u
C. formula mass of NaCl is the sum of atomic masses of Na and Cl.
D. both (a) and (c)

Answer
524.7k+ views
Hint: The three – dimensional arrangement of the atoms depicts the crystal lattice of any molecule. In a crystal, the arrangement of atoms is in a way that some atoms surround others. Formula mass of any compound is the sum of the atomic mass of total atoms present in that molecule. The lattice type of NaCl is Face centered cubic cell.
Complete answer:
The formula mass of any compound is the sum of the atomic masses when the atoms are in the bonded state. We have been given NaCl which is an ionic solid. It is formed in the stoichiometric ratio of 1 : 1 that means, 1 sodium atom$N{{a}^{+}}$is needed for combining by transferring the valence electron of 1 to the chlorine atom that forms $C{{l}^{-}}$. This means sodium is the cation and chlorine is the anion that balances each other’s charges and makes the NaCl molecule neutral.
Thus, the ionic solid sodium chloride exist as in the form of the crystal lattice in the FCC cubic cell that consist of lattice points that are the Na and Cl ions situated on all the faces and the corners of a cube making it a definite solid and without any single entity as $N{{a}^{+}}$or$C{{l}^{-}}$.
Therefore, the formula weight of NaCl will have the sum of the atomic masses of Na and Cl as:
Na + Cl = 23 + 35.5
Formula unit mass of NaCl = 58.5
Hence, the correct options are both (a) and (c) as the NaCl does not exist as a single entity and the formula unit mass consists of the mass of Na and Cl.
So, option D is correct.
Note:
In a crystal lattice, the smallest part of a crystal is called a unit cell. A crystal lattice consists of various lattice points, these points can be the ions or atoms of the molecule. Unit cells are classified as primitive (simple) and centered unit types that have FCC, BCC, and ECC. In NaCl the cation and anion is situated in the same number and has the same number of ions attached to each of them.
Complete answer:
The formula mass of any compound is the sum of the atomic masses when the atoms are in the bonded state. We have been given NaCl which is an ionic solid. It is formed in the stoichiometric ratio of 1 : 1 that means, 1 sodium atom$N{{a}^{+}}$is needed for combining by transferring the valence electron of 1 to the chlorine atom that forms $C{{l}^{-}}$. This means sodium is the cation and chlorine is the anion that balances each other’s charges and makes the NaCl molecule neutral.
Thus, the ionic solid sodium chloride exist as in the form of the crystal lattice in the FCC cubic cell that consist of lattice points that are the Na and Cl ions situated on all the faces and the corners of a cube making it a definite solid and without any single entity as $N{{a}^{+}}$or$C{{l}^{-}}$.
Therefore, the formula weight of NaCl will have the sum of the atomic masses of Na and Cl as:
Na + Cl = 23 + 35.5
Formula unit mass of NaCl = 58.5
Hence, the correct options are both (a) and (c) as the NaCl does not exist as a single entity and the formula unit mass consists of the mass of Na and Cl.
So, option D is correct.
Note:
In a crystal lattice, the smallest part of a crystal is called a unit cell. A crystal lattice consists of various lattice points, these points can be the ions or atoms of the molecule. Unit cells are classified as primitive (simple) and centered unit types that have FCC, BCC, and ECC. In NaCl the cation and anion is situated in the same number and has the same number of ions attached to each of them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




