
$ {P_4}{O_6} $ reacts with water to give:
(A) $ {H_3}P{O_3} $
(B) $ {H_2}{P_2}{O_7} $
(C) $ HP{O_3} $
(D) $ {H_3}P{O_4} $
Answer
547.5k+ views
Hint: Phosphorus trioxide reacts with hydrogen chloride to give phosphorous trichloride and phosphoric acid. In the given question we need to know the product formed when the $ {P_4}{O_6} $ reacts with water.
Complete step by step answer:
$ {P_4}{O_6} $ reacts with water to give phosphoric acid.
The chemical name of $ {P_4}{O_6} $ is Phosphorus trioxide.
The structure is:
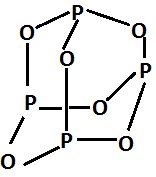
It is formed by burning phosphorus in the limited supply of oxygen. The reaction is as follows:
$ {P_4} + 3{O_2} \to {P_4}{O_6} $
This product reacts with water and forms phosphorus acid. The chemical formula of phosphoric acid is $ {H_3}P{O_3} $ The structure is:
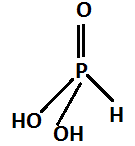
The reaction occurs as follows: $ {P_4}{O_6} + 6{H_2}O \to 4{H_3}P{O_3} $
Hence, option A. $ {H_3}P{O_3} $ is the correct answer to the given question.
Additional information:
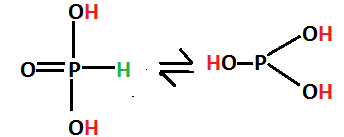
$ {H_3}P{O_3} $ exists in tautomeric form because they are readily convertible and the former is called phosphonic acid whereas the latter is called phosphoric acid.
Note:
Uses of $ {H_3}P{O_3} $ :
For the production of basic lead phosphite. It is used as a stabilizer in Polyvinyl chloride and other polymers containing chlorine.
As a reducing agent: it is a strong reducing agent which is also used in production of synthetic fibers and pesticides.
As a ligand: with the metals of $ {d^6} $ configuration, it acts as a ligand by forming a co-ordinate bond with those metals.
Complete step by step answer:
$ {P_4}{O_6} $ reacts with water to give phosphoric acid.
The chemical name of $ {P_4}{O_6} $ is Phosphorus trioxide.
The structure is:

It is formed by burning phosphorus in the limited supply of oxygen. The reaction is as follows:
$ {P_4} + 3{O_2} \to {P_4}{O_6} $
This product reacts with water and forms phosphorus acid. The chemical formula of phosphoric acid is $ {H_3}P{O_3} $ The structure is:

The reaction occurs as follows: $ {P_4}{O_6} + 6{H_2}O \to 4{H_3}P{O_3} $
Hence, option A. $ {H_3}P{O_3} $ is the correct answer to the given question.
Additional information:
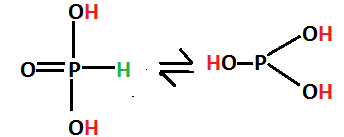
$ {H_3}P{O_3} $ exists in tautomeric form because they are readily convertible and the former is called phosphonic acid whereas the latter is called phosphoric acid.
Note:
Uses of $ {H_3}P{O_3} $ :
For the production of basic lead phosphite. It is used as a stabilizer in Polyvinyl chloride and other polymers containing chlorine.
As a reducing agent: it is a strong reducing agent which is also used in production of synthetic fibers and pesticides.
As a ligand: with the metals of $ {d^6} $ configuration, it acts as a ligand by forming a co-ordinate bond with those metals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




