
P in \[PC{l_5}\] has \[s{p^3}\] hybridization. Which one of the following statement is wrong about \[PC{l_5}\] structure?
A.Two \[P - Cl\] bonds are stronger and three \[P - Cl\] bonds are weaker.
B.Two \[P - Cl\] bonds are axial and larger than three \[P - Cl\] equatorial bond.
C.\[PC{l_5}\]has trigonal bipyramidal geometry with non- polar nature.
D.All the above statements are wrong.
Answer
512.1k+ views
Hint: The VSEPR theory can be used to predict the shape from the hybridization. The number of types of bonds present in phosphorus pentachloride is two which is axial bond and equatorial bond. And the hybridization of \[PC{l_5}\] is \[s{p^3}\]. Which means, two electrons are present in 3s orbital and three electrons are present in 3p orbital. The shorter bond is always stronger than the longer bond.
Complete answer:
Among the following statements, the first option, “Two \[P - Cl\] bonds are stronger and three \[P - Cl\] bonds are weaker “is a wrong statement. Because, the number of equatorial bonds present in \[PC{l_5}\] is three and the number of axial bonds is two. And the axial bonds are longer than equatorial bonds. We can draw the structure of \[PC{l_5}\],
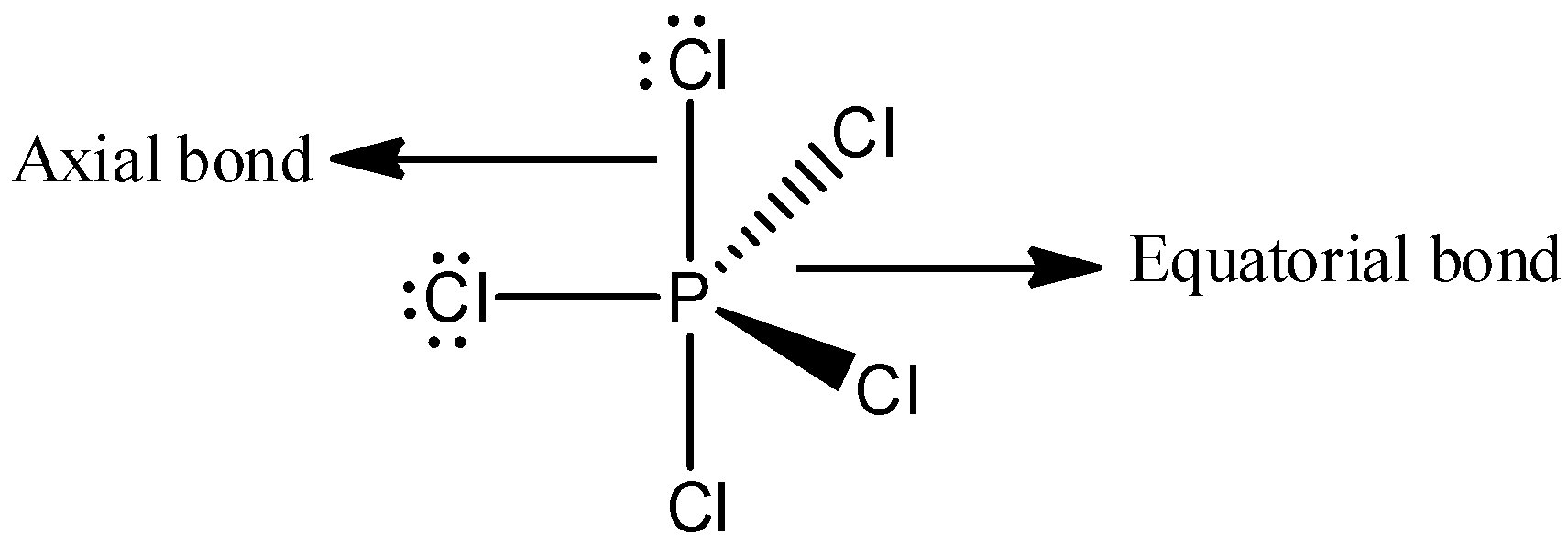
The bond length is always inversely proportional to the bond strength. Therefore, when the bond length is increased, its bond strength will decrease and here, the two \[P - Cl\] are weaker than three \[P - Cl\] bonds. Thus, we can say that the statement, “Two \[P - Cl\] bonds are stronger and three \[P - Cl\] bonds are weaker” is wrong.
Hence, option (A) is correct.
Two \[P - Cl\] bonds are axial and larger than three \[P - Cl\] equatorial bonds is a correct statement. Hence, the option (B) is incorrect.
The statement, \[PC{l_5}\] has trigonal bipyramidal geometry with non- polar nature is correct. Let’s draw the structure,
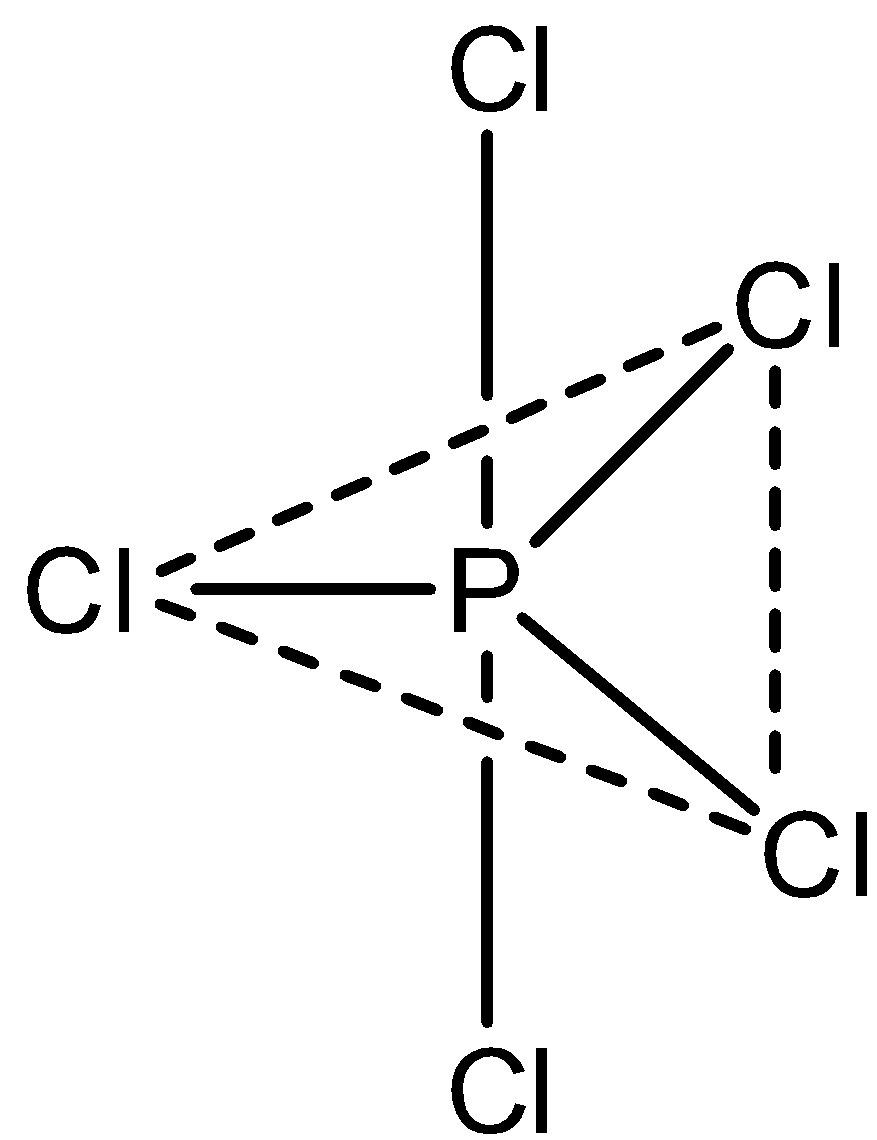
Hence, option (C) is incorrect.
Only the first statement is wrong and the other two statements are correct. Hence, the option (D) is incorrect.
Note:
Among the given statements, two \[P - Cl\] bonds are stronger and three \[P - Cl\] bonds are weaker is a wrong statement. Because, the axial bond present in phosphorus pentachloride is always weaker than the equatorial bond. There are two equatorial bonds present in \[PC{l_5}\] and that two bonds are weaker than the other three bonds. And the remaining two statements are true about \[PC{l_5}\] structure.
Complete answer:
Among the following statements, the first option, “Two \[P - Cl\] bonds are stronger and three \[P - Cl\] bonds are weaker “is a wrong statement. Because, the number of equatorial bonds present in \[PC{l_5}\] is three and the number of axial bonds is two. And the axial bonds are longer than equatorial bonds. We can draw the structure of \[PC{l_5}\],

The bond length is always inversely proportional to the bond strength. Therefore, when the bond length is increased, its bond strength will decrease and here, the two \[P - Cl\] are weaker than three \[P - Cl\] bonds. Thus, we can say that the statement, “Two \[P - Cl\] bonds are stronger and three \[P - Cl\] bonds are weaker” is wrong.
Hence, option (A) is correct.
Two \[P - Cl\] bonds are axial and larger than three \[P - Cl\] equatorial bonds is a correct statement. Hence, the option (B) is incorrect.
The statement, \[PC{l_5}\] has trigonal bipyramidal geometry with non- polar nature is correct. Let’s draw the structure,

Hence, option (C) is incorrect.
Only the first statement is wrong and the other two statements are correct. Hence, the option (D) is incorrect.
Note:
Among the given statements, two \[P - Cl\] bonds are stronger and three \[P - Cl\] bonds are weaker is a wrong statement. Because, the axial bond present in phosphorus pentachloride is always weaker than the equatorial bond. There are two equatorial bonds present in \[PC{l_5}\] and that two bonds are weaker than the other three bonds. And the remaining two statements are true about \[PC{l_5}\] structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




