
What is an ozonide in the mechanism for ozonide formation?
Answer
524.4k+ views
Hint: We have to know that the ozonide is the polyatomic anion $O_3^ - $. Cyclic
natural mixtures shaped by the expansion of ozone to an alkene are additionally, called ozonides. Inorganic ozonides are dim red salts. The anion has the bowed state of the ozone atom. Inorganic ozonides are shaped by consuming potassium, rubidium, or caesium in ozone, or by treating the salt metal hydroxide with ozone; if potassium is left undisturbed in air for quite a long time it collects a covering of superoxide and ozonide.
Complete step by step answer:
We have to see that ozonolysis is a natural response where the unsaturated obligations of alkenes, alkynes, or azo mixtures are cut with ozone. Alkenes and alkynes structure natural mixtures in which the different carbon–carbon bond has been supplanted by a carbonyl gathering while azo mixtures structure nitrosamines.
We have to know that i ozonide is the $1,2,4 - trioxolane$ structure that is shaped when ozone responds with an alkene,
The main middle in the response is known as a molozonide. A molozonide is a $1,2,4 - trioxolane$, (tri =three; oxa = oxygen; plane = soaked 5-membered ring).
The molozonide is flimsy. It quickly changes over in a progression of steps to an ozonide.
We have to know,ozonide is a $1,2,4 - trioxolane$. It quickly decays in water to frame carbonyl mixtures like aldehydes and ketones. It shows the development of the molozonide and ozonide intermediates as a component of the instrument.
The mechanism for the molozonide formation has to be formed, when ozonide reacts with alkene. That has to be given,
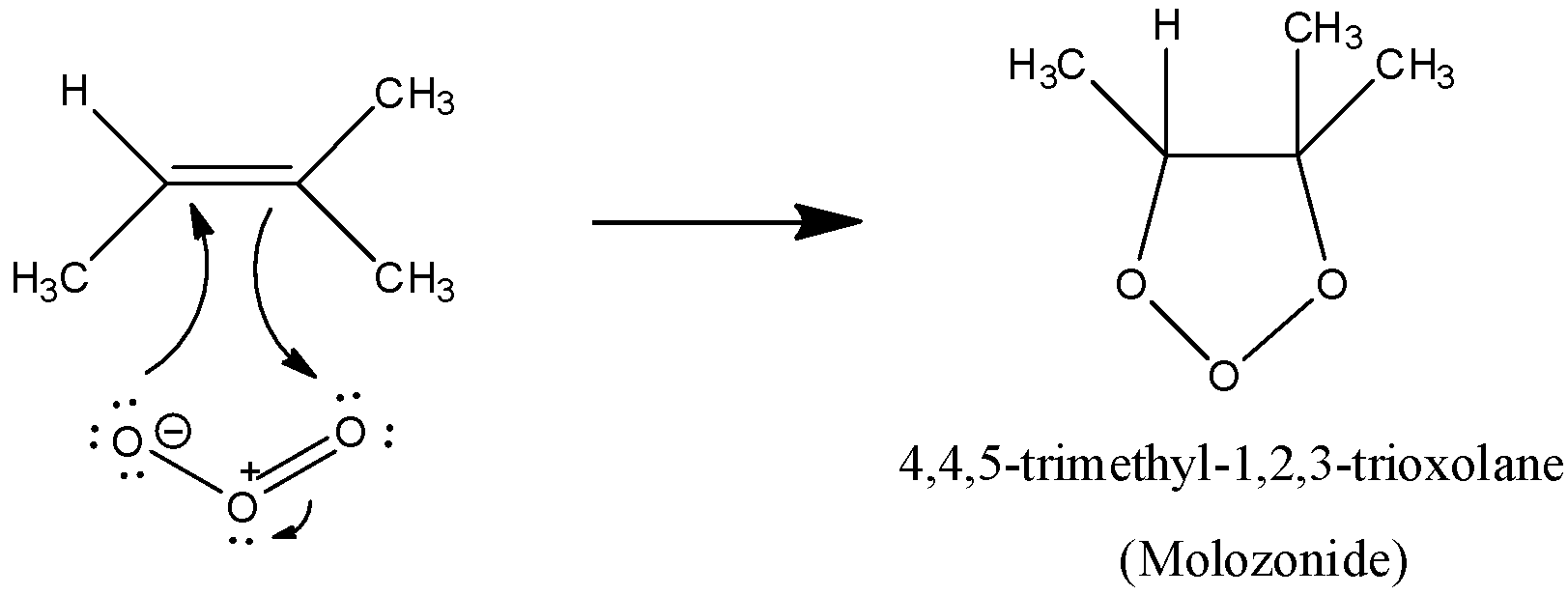
Note: We have to remember that the natural ozonides are called asmol ozonides and are normally framed by the expansion response among ozone and alkenes. They are infrequently separated over the span of the ozonolysis response grouping. Molozonides are temperamental and quickly convert to the trioxolane ring structure with a five-membered $C - O - O - C - O$ ring.
natural mixtures shaped by the expansion of ozone to an alkene are additionally, called ozonides. Inorganic ozonides are dim red salts. The anion has the bowed state of the ozone atom. Inorganic ozonides are shaped by consuming potassium, rubidium, or caesium in ozone, or by treating the salt metal hydroxide with ozone; if potassium is left undisturbed in air for quite a long time it collects a covering of superoxide and ozonide.
Complete step by step answer:
We have to see that ozonolysis is a natural response where the unsaturated obligations of alkenes, alkynes, or azo mixtures are cut with ozone. Alkenes and alkynes structure natural mixtures in which the different carbon–carbon bond has been supplanted by a carbonyl gathering while azo mixtures structure nitrosamines.
We have to know that i ozonide is the $1,2,4 - trioxolane$ structure that is shaped when ozone responds with an alkene,
The main middle in the response is known as a molozonide. A molozonide is a $1,2,4 - trioxolane$, (tri =three; oxa = oxygen; plane = soaked 5-membered ring).
The molozonide is flimsy. It quickly changes over in a progression of steps to an ozonide.
We have to know,ozonide is a $1,2,4 - trioxolane$. It quickly decays in water to frame carbonyl mixtures like aldehydes and ketones. It shows the development of the molozonide and ozonide intermediates as a component of the instrument.
The mechanism for the molozonide formation has to be formed, when ozonide reacts with alkene. That has to be given,

Note: We have to remember that the natural ozonides are called asmol ozonides and are normally framed by the expansion response among ozone and alkenes. They are infrequently separated over the span of the ozonolysis response grouping. Molozonides are temperamental and quickly convert to the trioxolane ring structure with a five-membered $C - O - O - C - O$ ring.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




