
Oxygen combines with nascent oxygen to form ozone.
a.) True
b.) False
Answer
606.6k+ views
Hint: Solve this question by understanding the basic equation involved in formation of ozone. The element in both atmospheric oxygen and ozone is O (atomic number – 8).
Complete step by step answer:
The structure of ozone is given as –
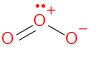
Ozone - \[{{\text{O}}_{\text{3}}}\], is an allotropic molecular form of oxygen containing three atoms of oxygen. It is generated by the passage of oxygen - \[{{\text{O}}_{\text{2}}}\] through a high voltage potential resulting in the attachment and formation of a third oxygen atom. It has a molar mass of 48 g/mol.
Naturally, Ozone is made when ultraviolet light strikes on oxygen, which breaks it into two atomic oxygen, i.e. nascent Oxygen (represented by O). Nascent Oxygen then combines with atmospheric oxygen to form ozone (step 2 of the reaction).
\[\begin{align}
& {{O}_{2}}+h{{\nu }_{uv}}\to 2O \\
& O+{{O}_{2}}\leftrightarrow {{O}_{3}} \\
\end{align}\]
Therefore, the answer is – option (a) – The given statement is true.
Additional information:
The term ‘ozone’ comes from the Greek word ‘ozein’ which means - ‘to smell’.
Note: Ozone layer is defined as, “A region in the earth’s stratosphere that contains high concentrations of ozone and protects the earth from harmful ultraviolet radiations of the sun.” Ozone layer is also known as ozone shield. It is present in Earth’s stratosphere. It is made up of a large amount of ozone - \[{{O}_{3}}\] . “Dobson Unit” is a measure of the amount of ozone present overhead.
Complete step by step answer:
The structure of ozone is given as –

Ozone - \[{{\text{O}}_{\text{3}}}\], is an allotropic molecular form of oxygen containing three atoms of oxygen. It is generated by the passage of oxygen - \[{{\text{O}}_{\text{2}}}\] through a high voltage potential resulting in the attachment and formation of a third oxygen atom. It has a molar mass of 48 g/mol.
Naturally, Ozone is made when ultraviolet light strikes on oxygen, which breaks it into two atomic oxygen, i.e. nascent Oxygen (represented by O). Nascent Oxygen then combines with atmospheric oxygen to form ozone (step 2 of the reaction).
\[\begin{align}
& {{O}_{2}}+h{{\nu }_{uv}}\to 2O \\
& O+{{O}_{2}}\leftrightarrow {{O}_{3}} \\
\end{align}\]
Therefore, the answer is – option (a) – The given statement is true.
Additional information:
The term ‘ozone’ comes from the Greek word ‘ozein’ which means - ‘to smell’.
Note: Ozone layer is defined as, “A region in the earth’s stratosphere that contains high concentrations of ozone and protects the earth from harmful ultraviolet radiations of the sun.” Ozone layer is also known as ozone shield. It is present in Earth’s stratosphere. It is made up of a large amount of ozone - \[{{O}_{3}}\] . “Dobson Unit” is a measure of the amount of ozone present overhead.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




