
How many oxygen atoms have $ - 1$ oxidation state in one mole product containing $Cr$?
${K_2}C{r_2}{O_7} + {H_2}S{O_4} + {H_2}{O_2}\xrightarrow[{{\text{ether}}}]{{{\text{low temperature}}}}$
Answer
513k+ views
Hint: The number of electrons gained or lost by an element of a compound while formation of a chemical bond is known as its oxidation state. In a chemical reaction, if the oxidation state of an element increases, then it is said to be oxidized while if the oxidation state decreases, then it is said to be reduced.
Complete answer: As per question, the given reaction is as follows:
${K_2}C{r_2}{O_7} + {H_2}S{O_4} + {H_2}{O_2}\xrightarrow[{{\text{ether}}}]{{{\text{low temperature}}}}$
It is a redox reaction in which potassium dichromate reacts with hydrogen peroxide and sulphuric acid and gets reduced to chromium (III) sulphate along with the formation of potassium sulphate, water and dioxygen. The reaction proceeds as follows:
${K_2}C{r_2}{O_7} + 4{H_2}S{O_4} + 3{H_2}{O_2}\xrightarrow[{{\text{ether}}}]{{{\text{low temperature}}}}C{r_2}{(S{O_4})_3} + 7{H_2}O + {K_2}S{O_4} + 3{O_2}$
The oxidation state of chromium in ${K_2}C{r_2}{O_7}$ is $ + 6$ whereas the oxidation state of chromium in $C{r_2}{(S{O_4})_3}$ is $ + 3$. Hence potassium dichromate gets reduced during the reaction process and acts as an oxidizing agent.
Now, the structure of $C{r_2}{(S{O_4})_3}$ is as follows:
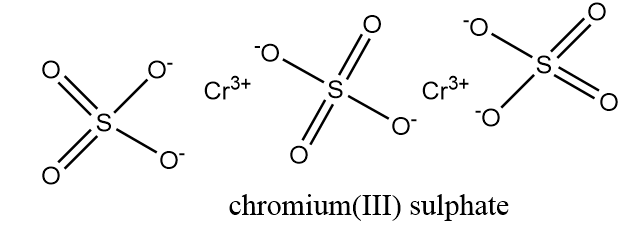
From the structure of $C{r_2}{(S{O_4})_3}$, it is clear that all the oxygen atoms connected via single bond will be present in their $ - 1$ oxidation state and all oxygen atoms connected to sulphur via double bond will be present in their $ - 2$ oxidation state.
Hence, the number of oxygen atoms have $ - 1$ oxidation state in one mole product containing $Cr = 6$.
Note:
It is important to note that trivalent chromium compounds are amphoteric in nature and chromium (III) sulphate generally exist in its hydrated form i.e., in the form of $C{r_2}{(S{O_4})_3}.x{H_2}O$ where the value of $x$ lies in the range of $0$ to $18$.
Complete answer: As per question, the given reaction is as follows:
${K_2}C{r_2}{O_7} + {H_2}S{O_4} + {H_2}{O_2}\xrightarrow[{{\text{ether}}}]{{{\text{low temperature}}}}$
It is a redox reaction in which potassium dichromate reacts with hydrogen peroxide and sulphuric acid and gets reduced to chromium (III) sulphate along with the formation of potassium sulphate, water and dioxygen. The reaction proceeds as follows:
${K_2}C{r_2}{O_7} + 4{H_2}S{O_4} + 3{H_2}{O_2}\xrightarrow[{{\text{ether}}}]{{{\text{low temperature}}}}C{r_2}{(S{O_4})_3} + 7{H_2}O + {K_2}S{O_4} + 3{O_2}$
The oxidation state of chromium in ${K_2}C{r_2}{O_7}$ is $ + 6$ whereas the oxidation state of chromium in $C{r_2}{(S{O_4})_3}$ is $ + 3$. Hence potassium dichromate gets reduced during the reaction process and acts as an oxidizing agent.
Now, the structure of $C{r_2}{(S{O_4})_3}$ is as follows:

From the structure of $C{r_2}{(S{O_4})_3}$, it is clear that all the oxygen atoms connected via single bond will be present in their $ - 1$ oxidation state and all oxygen atoms connected to sulphur via double bond will be present in their $ - 2$ oxidation state.
Hence, the number of oxygen atoms have $ - 1$ oxidation state in one mole product containing $Cr = 6$.
Note:
It is important to note that trivalent chromium compounds are amphoteric in nature and chromium (III) sulphate generally exist in its hydrated form i.e., in the form of $C{r_2}{(S{O_4})_3}.x{H_2}O$ where the value of $x$ lies in the range of $0$ to $18$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




