
How many oxygen atoms are present in a tablet weighing \[360mg\] of Aspirin (Aspirin-\[{C_9}{H_8}{O_4}\]).
Answer
572.1k+ views
Hint:Aspirin is an anti-inflammatory drug used for relieving pain, inflammation and fever. The number of oxygen atoms can be determined using the mole concept.
Complete step by step answer:Aspirin is an organic compound also known as acetylsalicylic acid. The molecular formula of aspirin is \[{C_9}{H_8}{O_4}\]. It is an aromatic compound containing a benzene ring. The functional groups attached to the benzene ring are one carboxylic (\[COOH\]) group and one acetyl (\[COC{H_3}\]) group. The structure of aspirin is
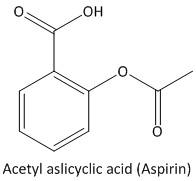
The molecular or chemical formula of Aspirin is \[{C_9}{H_8}{O_4}\]. The molecular weight of Aspirin is =
\[9\] x atomic mass of \[C\] atom + \[8\] x atomic mass of \[H\] atom + \[4\] x atomic mass of \[O\] atom
=$9 \times 12 + 8 \times 1 + 4 \times 16 = 180g$
A tablet of Aspirin weighs \[360mg\]. Thus the moles of Aspirin contain in \[360mg\] of Aspirin is,
$moles = \dfrac{{weight}}{{molar{\text{ }}mass}}$
$ = \dfrac{{360 \times {{10}^{ - 3}}}}{{180}} = 2 \times {10^{ - 3}}$
One molecule of Aspirin contains four molecules of oxygen atom. Thus the moles of oxygen in \[2{\text{ }} \times {10^{ - 3}}\] moles of Aspirin = $4 \times 2 \times {10^{ - 3}} = 8 \times {10^{ - 3}}$
According to Avogadro’s law, one gram mole of a substance contains \[6.023 \times {10^{23}}\] atoms. Here the number of moles of oxygen is \[8 \times {10^{ - 3}}\]. Therefore the number of oxygen atoms present in \[8{\text{ }} \times {10^{ - 3}}\] moles of oxygen = moles of oxygen x Avogadro’s number
= $8 \times {10^{ - 3}} \times 6.023 \times {10^{23}} = 4.81 \times {10^{21}}$
Hence \[4.81 \times {10^{21}}\] number of oxygen atoms is present in a tablet weighing \[360mg\] of Aspirin (Aspirin-\[{C_9}{H_8}{O_4}\]).
Note:Before the discovery of aspirin, \[2\] -hydroxy benzoic acid was used as a drug. This leads to severe irritation in the stomach after consumption. In order to avoid such irritation the hydroxyl group was protected with acetyl group.
Complete step by step answer:Aspirin is an organic compound also known as acetylsalicylic acid. The molecular formula of aspirin is \[{C_9}{H_8}{O_4}\]. It is an aromatic compound containing a benzene ring. The functional groups attached to the benzene ring are one carboxylic (\[COOH\]) group and one acetyl (\[COC{H_3}\]) group. The structure of aspirin is

The molecular or chemical formula of Aspirin is \[{C_9}{H_8}{O_4}\]. The molecular weight of Aspirin is =
\[9\] x atomic mass of \[C\] atom + \[8\] x atomic mass of \[H\] atom + \[4\] x atomic mass of \[O\] atom
=$9 \times 12 + 8 \times 1 + 4 \times 16 = 180g$
A tablet of Aspirin weighs \[360mg\]. Thus the moles of Aspirin contain in \[360mg\] of Aspirin is,
$moles = \dfrac{{weight}}{{molar{\text{ }}mass}}$
$ = \dfrac{{360 \times {{10}^{ - 3}}}}{{180}} = 2 \times {10^{ - 3}}$
One molecule of Aspirin contains four molecules of oxygen atom. Thus the moles of oxygen in \[2{\text{ }} \times {10^{ - 3}}\] moles of Aspirin = $4 \times 2 \times {10^{ - 3}} = 8 \times {10^{ - 3}}$
According to Avogadro’s law, one gram mole of a substance contains \[6.023 \times {10^{23}}\] atoms. Here the number of moles of oxygen is \[8 \times {10^{ - 3}}\]. Therefore the number of oxygen atoms present in \[8{\text{ }} \times {10^{ - 3}}\] moles of oxygen = moles of oxygen x Avogadro’s number
= $8 \times {10^{ - 3}} \times 6.023 \times {10^{23}} = 4.81 \times {10^{21}}$
Hence \[4.81 \times {10^{21}}\] number of oxygen atoms is present in a tablet weighing \[360mg\] of Aspirin (Aspirin-\[{C_9}{H_8}{O_4}\]).
Note:Before the discovery of aspirin, \[2\] -hydroxy benzoic acid was used as a drug. This leads to severe irritation in the stomach after consumption. In order to avoid such irritation the hydroxyl group was protected with acetyl group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




