
Oxidation state of oxygen in $Cr{O_5}$ is.
A)$ - 1$
B)$ - 2$.
C) Both (1) and (2).
D)$ - 1/2$.
Answer
589.8k+ views
Hint: First we have to find the oxidation number of chromium and then count the number of peroxide bonds and double bonded oxygen and then we can calculate the oxidation of chromium.
Complete step by step answer:
Now, we see the structure of$Cr{O_5}$.
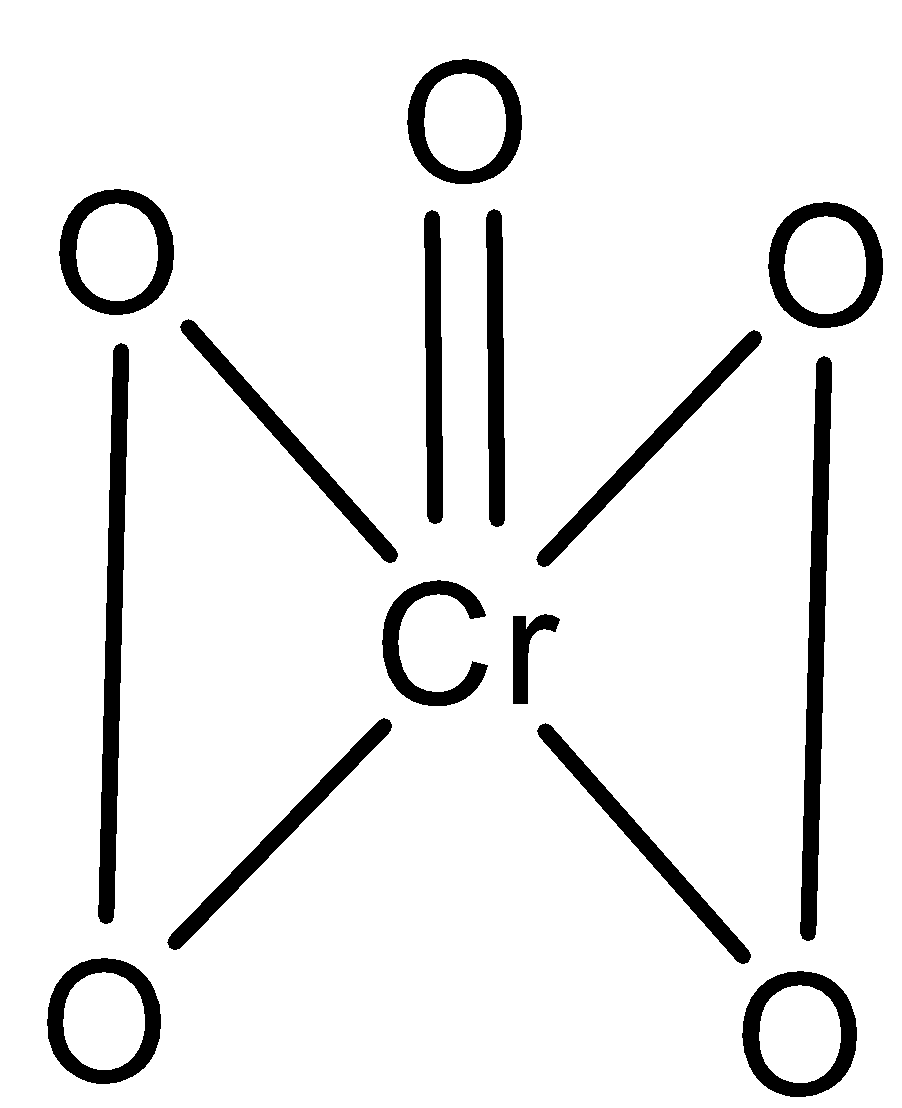
It is a butterfly structure in which the central chromium is surrounded by 4 peroxy cyclic oxygen and one coordinate oxygen.
In$Cr{O_5}$, one chromium atom is attached to five oxygen atoms and it makes double bonds with the one oxygen atom and single bond with the other four oxygen atoms.
The oxidation number of oxygen which are connected to chromium through single bond is $ - 1$ and the oxidation number of oxygen attached through the double bond is $ - 2.$
Thus, an option C is correct.
Note: We know that oxidation state is the loss of an electron in a chemical compound. We can now see a few rules of oxidation numbers.
1.A free element will be zero as its oxidation number.
2.Monoatomic ions will have an oxidation number equal to charge of the ion.
3.In hydrogen, the oxidation number is ${\text{ + 1,}}$ when combined with elements having less 4.electronegativity, the oxidation number of hydrogen is -1.
5.In compounds of oxygen, the oxidation number of oxygen will be -2 and in peroxides it will be -1.
6.Group 1 elements will have +1 oxidation number.
7.Group 2 elements will have +2 oxidation number.
8.Group 17 elements will have -1 oxidation number.
9Sum of oxidation numbers of all atoms in neutral compounds is zero.
In a polyatomic ion, the sum of the oxidation number is equal to the charge of the ion.
Complete step by step answer:
Now, we see the structure of$Cr{O_5}$.

It is a butterfly structure in which the central chromium is surrounded by 4 peroxy cyclic oxygen and one coordinate oxygen.
In$Cr{O_5}$, one chromium atom is attached to five oxygen atoms and it makes double bonds with the one oxygen atom and single bond with the other four oxygen atoms.
The oxidation number of oxygen which are connected to chromium through single bond is $ - 1$ and the oxidation number of oxygen attached through the double bond is $ - 2.$
Thus, an option C is correct.
Note: We know that oxidation state is the loss of an electron in a chemical compound. We can now see a few rules of oxidation numbers.
1.A free element will be zero as its oxidation number.
2.Monoatomic ions will have an oxidation number equal to charge of the ion.
3.In hydrogen, the oxidation number is ${\text{ + 1,}}$ when combined with elements having less 4.electronegativity, the oxidation number of hydrogen is -1.
5.In compounds of oxygen, the oxidation number of oxygen will be -2 and in peroxides it will be -1.
6.Group 1 elements will have +1 oxidation number.
7.Group 2 elements will have +2 oxidation number.
8.Group 17 elements will have -1 oxidation number.
9Sum of oxidation numbers of all atoms in neutral compounds is zero.
In a polyatomic ion, the sum of the oxidation number is equal to the charge of the ion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




