
What is the oxidation number of sulphur in thiosulphuric acid, ${{H}_{2}}{{S}_{2}}{{O}_{3}}$?
Answer
494.1k+ views
Hint: Oxidation number of any atom is the total number of electrons that it may either gain or lose in order to form a chemical bond with another atom. It is also known as the oxidation state and it may be positive according to the type of the atom it is bonded with.
Complete answer:
Some basic rules for assigning oxidation number to any atom are as follows:
1. The oxidation number of a free element or a diatomic molecule is always zero.
2. The oxidation number of a mono-atomic ion is equal to the charge present on the ion.
3. The general oxidation number of hydrogen atoms is $+1$. But in the case of metal hydrides, it exists in its $-1$ oxidation state.
4. The general oxidation number of oxygen atoms is $-2$. But in the case of peroxides, it exists in its $-1$ oxidation state.
5. The oxidation number of alkali metals is $+1$ and alkaline earth metals is $+2$.
6. The oxidation number for halogens is $-1$, except when the element is bonded to the atom with higher electronegativity.
7. The sum of oxidation numbers of all the atoms is zero for a neutral compound.
8. For a polyatomic ion, the sum of oxidation number of each element is equal to the overall charge of the ion.
Now, we need to find oxidation number of sulphur in thiosulphuric acid i.e., ${{H}_{2}}{{S}_{2}}{{O}_{3}}$ which is structurally represented as follows:
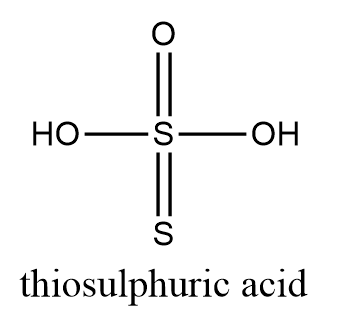
As we know the general oxidation number of the sulphur atom is $-2$. So, the oxidation number of terminal sulphur is $-2$. In case of a central sulphur atom, let the oxidation number of the sulphur atom be $x$ and we know that oxidation number of hydroxyl group is $-1$ and for oxygen atom is $-2$
$2\times (-1)+x+(-2)-2=0$
$\Rightarrow x=+6$
Hence, the oxidation numbers of terminal sulphur and central sulphur in thiosulphuric acid are $-2$ and $+6$ respectively.
Note:
It is important to note that along with the $-2$ and $+6$ oxidation states, the sulphur atom also exists in $0$, $+2$ and $+4$ oxidation states. The valence shell configuration of sulphur is $3{{s}^{2}}3{{p}^{4}}$ and due to presence of vacant d orbital, it has the tendency to show variable oxidation state. Remember, an oxygen atom does not have a vacant d-orbital, so it cannot exist in a $+6$ oxidation state.
Complete answer:
Some basic rules for assigning oxidation number to any atom are as follows:
1. The oxidation number of a free element or a diatomic molecule is always zero.
2. The oxidation number of a mono-atomic ion is equal to the charge present on the ion.
3. The general oxidation number of hydrogen atoms is $+1$. But in the case of metal hydrides, it exists in its $-1$ oxidation state.
4. The general oxidation number of oxygen atoms is $-2$. But in the case of peroxides, it exists in its $-1$ oxidation state.
5. The oxidation number of alkali metals is $+1$ and alkaline earth metals is $+2$.
6. The oxidation number for halogens is $-1$, except when the element is bonded to the atom with higher electronegativity.
7. The sum of oxidation numbers of all the atoms is zero for a neutral compound.
8. For a polyatomic ion, the sum of oxidation number of each element is equal to the overall charge of the ion.
Now, we need to find oxidation number of sulphur in thiosulphuric acid i.e., ${{H}_{2}}{{S}_{2}}{{O}_{3}}$ which is structurally represented as follows:

As we know the general oxidation number of the sulphur atom is $-2$. So, the oxidation number of terminal sulphur is $-2$. In case of a central sulphur atom, let the oxidation number of the sulphur atom be $x$ and we know that oxidation number of hydroxyl group is $-1$ and for oxygen atom is $-2$
$2\times (-1)+x+(-2)-2=0$
$\Rightarrow x=+6$
Hence, the oxidation numbers of terminal sulphur and central sulphur in thiosulphuric acid are $-2$ and $+6$ respectively.
Note:
It is important to note that along with the $-2$ and $+6$ oxidation states, the sulphur atom also exists in $0$, $+2$ and $+4$ oxidation states. The valence shell configuration of sulphur is $3{{s}^{2}}3{{p}^{4}}$ and due to presence of vacant d orbital, it has the tendency to show variable oxidation state. Remember, an oxygen atom does not have a vacant d-orbital, so it cannot exist in a $+6$ oxidation state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




