
What is the oxidation number of sulphur in ${ H }_{ 2 }SO_{ 4 }$?
Answer
609.6k+ views
Hint: Assume the oxidation number of sulphur to be X and then try to add oxidation numbers of all atoms present, they all should be equal to zero. Now find the value of X.
Complete step by step answer:
Structure of ${ H }_{ 2 }SO_{ 4 }$ -
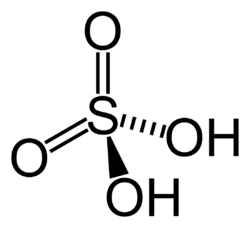
Oxidation number - Oxidation number, also called Oxidation State, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom.
We know that, because there is no charge given, sulfuric acid in this form is a neutral compound. When a compound is neutral, we know the oxidation numbers must add to 0.
In sulphuric acid, the charge on hydrogen is positive 1, and charge on oxygen is negative 2.
Since there are two hydrogen atoms therefore, net positive charge will be the number of atoms on each positive atom.
Thus, total positive charge = 2 (+1) = +2
Similarly, net negative charge will be the number of oxygen atoms multiplied by charge on each oxygen atom.
Thus, total negative charge = 4(-2) = -8
As the overall sulphuric acid molecule is neutral so, the net charge on the molecule will be zero. Therefore, calculate oxidation number of sulphur as follows.
Thus, the oxidation number of sulfur in ${ H }_{ 2 }SO_{ 4 }$ is +6.
Note: The only consistent variation of oxidation numbers with the periodic Table is that, in their compounds, Group 1 metals are always +1 and Group 2 metals are always +2.
The transition metals usually have more than one oxidation state.
Complete step by step answer:
Structure of ${ H }_{ 2 }SO_{ 4 }$ -

Oxidation number - Oxidation number, also called Oxidation State, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom.
We know that, because there is no charge given, sulfuric acid in this form is a neutral compound. When a compound is neutral, we know the oxidation numbers must add to 0.
In sulphuric acid, the charge on hydrogen is positive 1, and charge on oxygen is negative 2.
Since there are two hydrogen atoms therefore, net positive charge will be the number of atoms on each positive atom.
Thus, total positive charge = 2 (+1) = +2
Similarly, net negative charge will be the number of oxygen atoms multiplied by charge on each oxygen atom.
Thus, total negative charge = 4(-2) = -8
As the overall sulphuric acid molecule is neutral so, the net charge on the molecule will be zero. Therefore, calculate oxidation number of sulphur as follows.
Thus, the oxidation number of sulfur in ${ H }_{ 2 }SO_{ 4 }$ is +6.
Note: The only consistent variation of oxidation numbers with the periodic Table is that, in their compounds, Group 1 metals are always +1 and Group 2 metals are always +2.
The transition metals usually have more than one oxidation state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




