
What is the oxidation number of phosphorus in the ${H_3}P{O_4}$ molecule?
Answer
516k+ views
Hint: Before going through the question let us first talk about the oxidation state. The total number of electrons that an atom receives or loses in order to form a chemical bond with another atom is known as the oxidation number, also known as the oxidation state.
Complete answer: We know that oxidation state of an element is known as the charge which an atom the element has in its ion or appear to have when present in the combined state with other atoms, oxidation state is also known as oxidation number. For finding the oxidation number of an element we have to follow some rules that are the oxidation numbers of all the atoms in their elemental state.
Now, we have a general overview of oxidation state
Now, let us calculate the oxidation state of phosphorus in ${H_3}P{O_4}$ . The ${H_3}P{O_4}$ has following structure:-
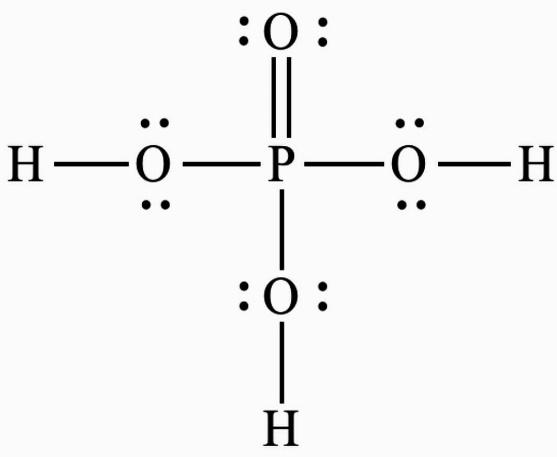
Phosphoric acid is a compound in which hydrogen is bound to a non-metal. Inside a compound, hydrogen has a \[ + 1\] charge while oxygen has a \[ - 2\] charge due to such bonding. The total charge of the given compound is zero since it is neutral.
The following equation must be written to determine the oxidation number of P:\[3{\text{ }}H{\text{ }} + {\text{ }}1{\text{ }}P{\text{ }} + {\text{ }}4{\text{ }}O{\text{ }} = {\text{ }}0\]
When the charges in the above equation are substituted, the result is
\[\begin{array}{*{20}{l}}
{3{\text{ }}\left( { + 1} \right){\text{ }} + {\text{ }}1{\text{ }}\left( X \right){\text{ }} + {\text{ }}4{\text{ }}\left( { - 2} \right){\text{ }} = {\text{ }}0} \\
{3{\text{ }} + {\text{ }}X{\text{ }} - 8{\text{ }} = {\text{ }}0} \\
{X{\text{ }} - 5{\text{ }} = {\text{ }}0} \\
{X{\text{ }} = {\text{ }} + 5.} \\
\end{array}\]
As a result, the oxidation number of phosphorus (P) in ${H_3}P{O_4}$ is determined to be \[ + 5.\]
Note:
And in its peroxide compounds, where its oxidation state is $ - 1$, oxygen usually reveals the oxidation state $ - 2$. As a result, we must first draw the compound structure before attempting to answer questions of this kind.
Complete answer: We know that oxidation state of an element is known as the charge which an atom the element has in its ion or appear to have when present in the combined state with other atoms, oxidation state is also known as oxidation number. For finding the oxidation number of an element we have to follow some rules that are the oxidation numbers of all the atoms in their elemental state.
Now, we have a general overview of oxidation state
Now, let us calculate the oxidation state of phosphorus in ${H_3}P{O_4}$ . The ${H_3}P{O_4}$ has following structure:-

Phosphoric acid is a compound in which hydrogen is bound to a non-metal. Inside a compound, hydrogen has a \[ + 1\] charge while oxygen has a \[ - 2\] charge due to such bonding. The total charge of the given compound is zero since it is neutral.
The following equation must be written to determine the oxidation number of P:\[3{\text{ }}H{\text{ }} + {\text{ }}1{\text{ }}P{\text{ }} + {\text{ }}4{\text{ }}O{\text{ }} = {\text{ }}0\]
When the charges in the above equation are substituted, the result is
\[\begin{array}{*{20}{l}}
{3{\text{ }}\left( { + 1} \right){\text{ }} + {\text{ }}1{\text{ }}\left( X \right){\text{ }} + {\text{ }}4{\text{ }}\left( { - 2} \right){\text{ }} = {\text{ }}0} \\
{3{\text{ }} + {\text{ }}X{\text{ }} - 8{\text{ }} = {\text{ }}0} \\
{X{\text{ }} - 5{\text{ }} = {\text{ }}0} \\
{X{\text{ }} = {\text{ }} + 5.} \\
\end{array}\]
As a result, the oxidation number of phosphorus (P) in ${H_3}P{O_4}$ is determined to be \[ + 5.\]
Note:
And in its peroxide compounds, where its oxidation state is $ - 1$, oxygen usually reveals the oxidation state $ - 2$. As a result, we must first draw the compound structure before attempting to answer questions of this kind.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




