
Out of ${H_2}{S_2}{O_3},{H_2}{S_4}{O_6},{H_2}S{O_5}{\text{ and }}{H_2}{S_2}{O_8}$ peroxy acids are:
A. ${H_2}{S_2}{O_3},{H_2}{S_4}{O_6}$
B. ${H_2}{S_4}{O_6},{H_2}S{O_5}$
C. ${H_2}S{O_5}{\text{, }}{H_2}{S_2}{O_8}$
D. ${H_2}{S_2}{O_3},{H_2}{S_2}{O_8}$
Answer
585.6k+ views
Hint: Peroxy acids are those which contain an acidic group $ - OOH$ that is replaced by the $ - OH$ group of an oxyacid. Peroxy acids are frequently used as an oxidant and are also known as peracid.
Complete answer: To find the proxy acid out of the options given we will start by analysing the structures of each compound.
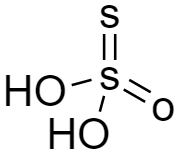
As per the structure of thiosulphuric acid (${H_2}{S_2}{O_3}$) is given below, we can see there is no $ - OOH$ group hence it is not peroxy acid.
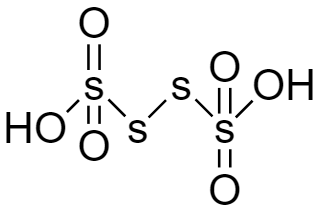
For the structure of Tetrathionic acid (${H_2}{S_4}{O_6}$) given below we can see there is no $ - OOH$ group hence it is also not a peroxy acid.
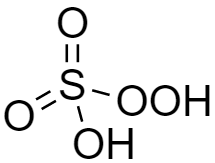
The structure of peroxymonosulfuric acid (${H_2}S{O_5}$) is shown below and we can see there is the presence of one $ - OOH$ group. Hence it is a peroxy acid. This is also known as Caro's acid.
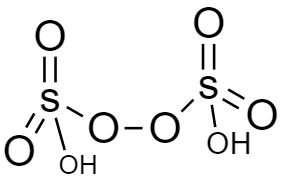
In the structure of peroxodisulfuric acid (${H_2}{S_2}{O_8}$), there is peroxy linkage hence this is also peroxy acid. This is also known as marshall’s acid. The structure is given below:
So, the correct answer is “Option C”.
Additional Information: Caro's acid i.e. peroxymonosulfuric acid is probably the most important inorganic peroxy acid, at least in terms of its production scale. It is used for the bleaching of pulp and the detoxification of cyanide in the mining industries. It is produced by treating sulphuric acid with hydrogen peroxide.
Note: Peroxide acid is generally prepared by reaction of the oxy acid with hydrogen peroxide, some of the sulphuric or other strong acids are often used to accelerate the reaction of weak oxy acid. They readily add oxygen to alkenes to give epoxides and are used to convert ketones into ester and amines into the nitro compound, amine oxides, or nitroso compounds.
Complete answer: To find the proxy acid out of the options given we will start by analysing the structures of each compound.
As per the structure of thiosulphuric acid (${H_2}{S_2}{O_3}$) is given below, we can see there is no $ - OOH$ group hence it is not peroxy acid.

For the structure of Tetrathionic acid (${H_2}{S_4}{O_6}$) given below we can see there is no $ - OOH$ group hence it is also not a peroxy acid.

The structure of peroxymonosulfuric acid (${H_2}S{O_5}$) is shown below and we can see there is the presence of one $ - OOH$ group. Hence it is a peroxy acid. This is also known as Caro's acid.

In the structure of peroxodisulfuric acid (${H_2}{S_2}{O_8}$), there is peroxy linkage hence this is also peroxy acid. This is also known as marshall’s acid. The structure is given below:

So, the correct answer is “Option C”.
Additional Information: Caro's acid i.e. peroxymonosulfuric acid is probably the most important inorganic peroxy acid, at least in terms of its production scale. It is used for the bleaching of pulp and the detoxification of cyanide in the mining industries. It is produced by treating sulphuric acid with hydrogen peroxide.
Note: Peroxide acid is generally prepared by reaction of the oxy acid with hydrogen peroxide, some of the sulphuric or other strong acids are often used to accelerate the reaction of weak oxy acid. They readily add oxygen to alkenes to give epoxides and are used to convert ketones into ester and amines into the nitro compound, amine oxides, or nitroso compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




