
Out of first 100 elements, numbers of elements having electrons in 3d orbitals (in their complete electronic configurations) are:
a.) 80
b.) 100
c.) 40
d.) 60
Answer
573k+ views
Hint: According to (n+l) rule filling of electrons in 3d orbitals starts once the 4s orbit get completely filled and we know that the 4s orbit gets completely filled when we complete the electron filling in the calcium atom. So all the elements whose atomic number is higher than Calcium will have 3d electrons in their 3rd orbit.
Complete Solution :
According to n + l rule electrons will always be filled first in the orbital whose n+l value is less and if for two orbitals n + l values will become equal then electrons will be filled first in the orbital whose n value is less.
- Here n is the number of energy levels or orbit. For 1s it will be 1 and for 3p it will be 3.
l is the value of azimuthal quantum number whose value is fix for each subshell
s = 0
p = 1
d = 2
f = 3
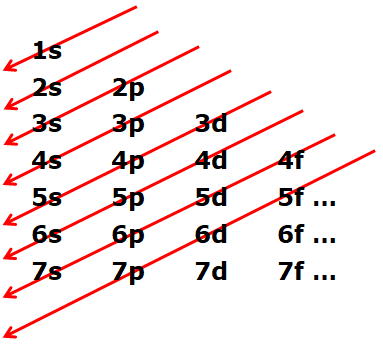
- This is the order to fill electrons into the atom. Starting from the 1s, we start from the tail of the arrow and move towards its head. Once reaching the head, we once again follow the tail of the next arrow.
So order of electron filling will be,
1s, 2s, 2s, 3s, 3p, 4s, 3d, 4p…………………..
Interesting observation is, first electrons are getting filled in 4s and after that in 3d.
n+l value for 3d= 3 + 2
= 5
n+l value for 4s= 4 + 0
= 4
So clearly 4s has a lesser value of n+l that’s why electrons will be filled in 4s orbital first.
- Calcium atomic number is 20 and its electronic configuration is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}$
- As maximum two electrons can be there in 4s orbitals so it is filled now.
From atomic number 21, electrons will be filled in 3d orbitals.
So from 21 to 100 we will be having 80 elements that have electrons in 3d orbitals.
So, the correct answer is “Option A”.
Additional Information:
Electron filling in the atoms is done according to aufbau principle which has 3 rules in it:
Pauli’s exclusion principle
Hund's maximum multiplicity rule
n+l rule
Note: Total 118 elements have been identified. The first 94 occur naturally on Earth, and the remaining 24 are synthetic elements produced in nuclear reactions. Out of 118, 95 are metals, 17 are non-metals and 6 are metalloids. Also total 37 elements in the periodic table are radioactive.
Complete Solution :
According to n + l rule electrons will always be filled first in the orbital whose n+l value is less and if for two orbitals n + l values will become equal then electrons will be filled first in the orbital whose n value is less.
- Here n is the number of energy levels or orbit. For 1s it will be 1 and for 3p it will be 3.
l is the value of azimuthal quantum number whose value is fix for each subshell
s = 0
p = 1
d = 2
f = 3

- This is the order to fill electrons into the atom. Starting from the 1s, we start from the tail of the arrow and move towards its head. Once reaching the head, we once again follow the tail of the next arrow.
So order of electron filling will be,
1s, 2s, 2s, 3s, 3p, 4s, 3d, 4p…………………..
Interesting observation is, first electrons are getting filled in 4s and after that in 3d.
n+l value for 3d= 3 + 2
= 5
n+l value for 4s= 4 + 0
= 4
So clearly 4s has a lesser value of n+l that’s why electrons will be filled in 4s orbital first.
- Calcium atomic number is 20 and its electronic configuration is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}$
- As maximum two electrons can be there in 4s orbitals so it is filled now.
From atomic number 21, electrons will be filled in 3d orbitals.
So from 21 to 100 we will be having 80 elements that have electrons in 3d orbitals.
So, the correct answer is “Option A”.
Additional Information:
Electron filling in the atoms is done according to aufbau principle which has 3 rules in it:
Pauli’s exclusion principle
Hund's maximum multiplicity rule
n+l rule
Note: Total 118 elements have been identified. The first 94 occur naturally on Earth, and the remaining 24 are synthetic elements produced in nuclear reactions. Out of 118, 95 are metals, 17 are non-metals and 6 are metalloids. Also total 37 elements in the periodic table are radioactive.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




