
Out of benzene, m-dinitrobenzene, and toluene which will undergo nitration most easily and why.
Answer
595.2k+ views
Hint: Nitration means addition of nitronium ion (\[NO_{2}^{+}\]) and it is a best example for electrophilic addition reaction. Nitronium ion is an electrophile and tries to attach to the molecule where a large number of electrons or high electron density is present.
Complete step by step answer:
In the question it is given that, out of benzene, m-dinitrobenzene, and toluene which will undergo nitration most easily.
First we should know the structures of the given compounds.
The structures of the given compounds are as follows.
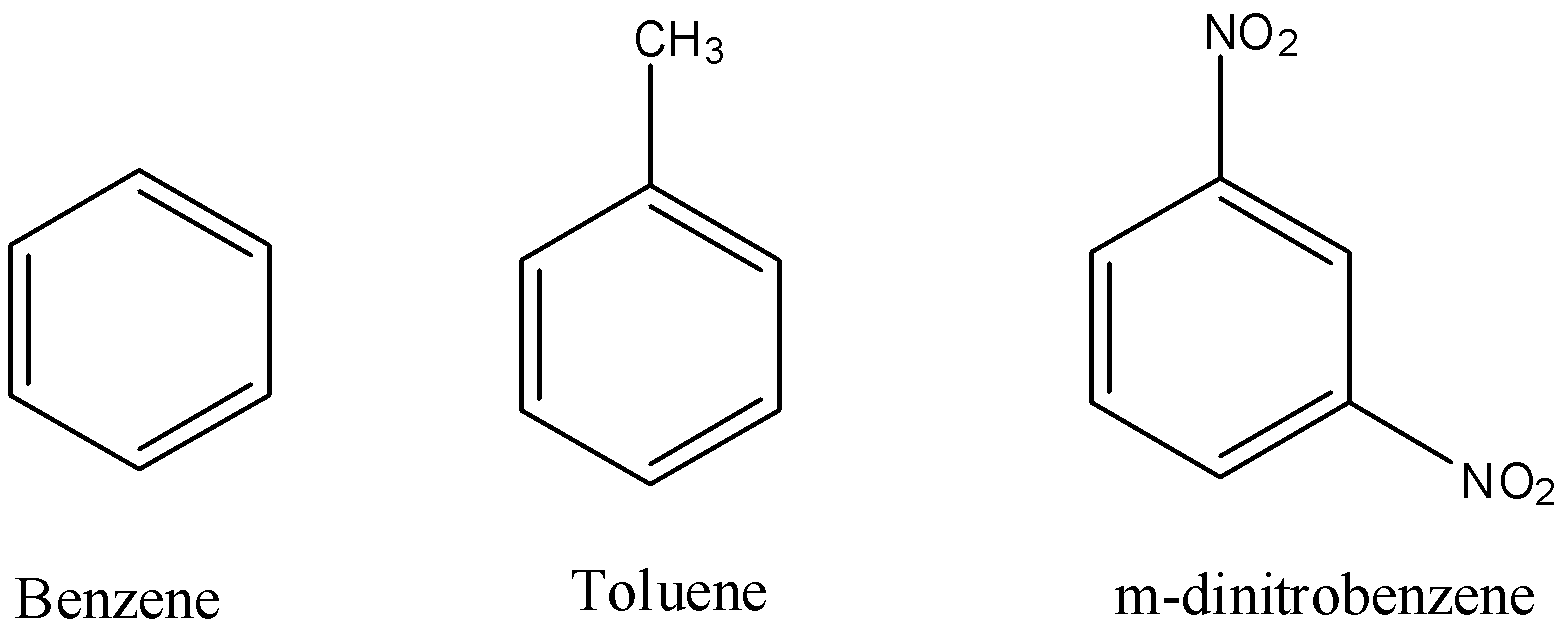
We know that the ease of nitration depends on the presence of electron density on the given compound to form the respective nitrate derivatives.
We know that Benzene is electron rich in nature but all the electrons are in resonance, so benzene itself won't easily donate electrons or won't easily undergo nitration reaction to form nitro derivative.
Toluene is also called methyl-benzene. Methyl group in toluene makes it an electron donating molecule.
So, ease of nitration is very easy in case of toluene when compared to simple benzene.
Coming to m-dinitrobenzene, the presence of two nitro groups makes the molecule as electron deficient. So, nitration in m-dinitrobenzene is very difficult to do.
Hence, the decreasing order of nitration of the given compounds is as follows:
Toluene > Benzene > m-dinitrobenzene
Note: Don’t be confused with nitro benzene and m-dinitrobenzene.
In nitro benzene only one nitro group will be there but in m-dinitrobenzene there are nitro groups in meta position to each other.
The structure of nitro benzene is
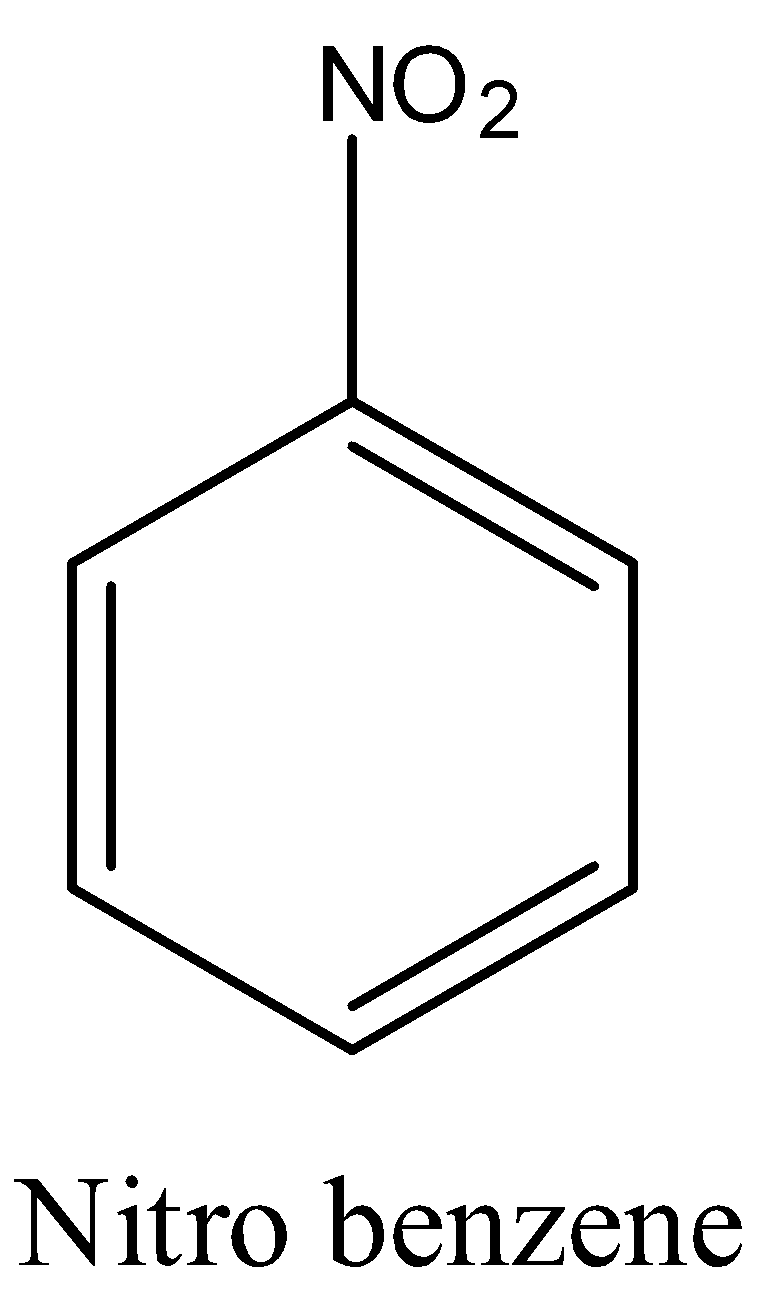
The structure of m-dinitrobenzene is
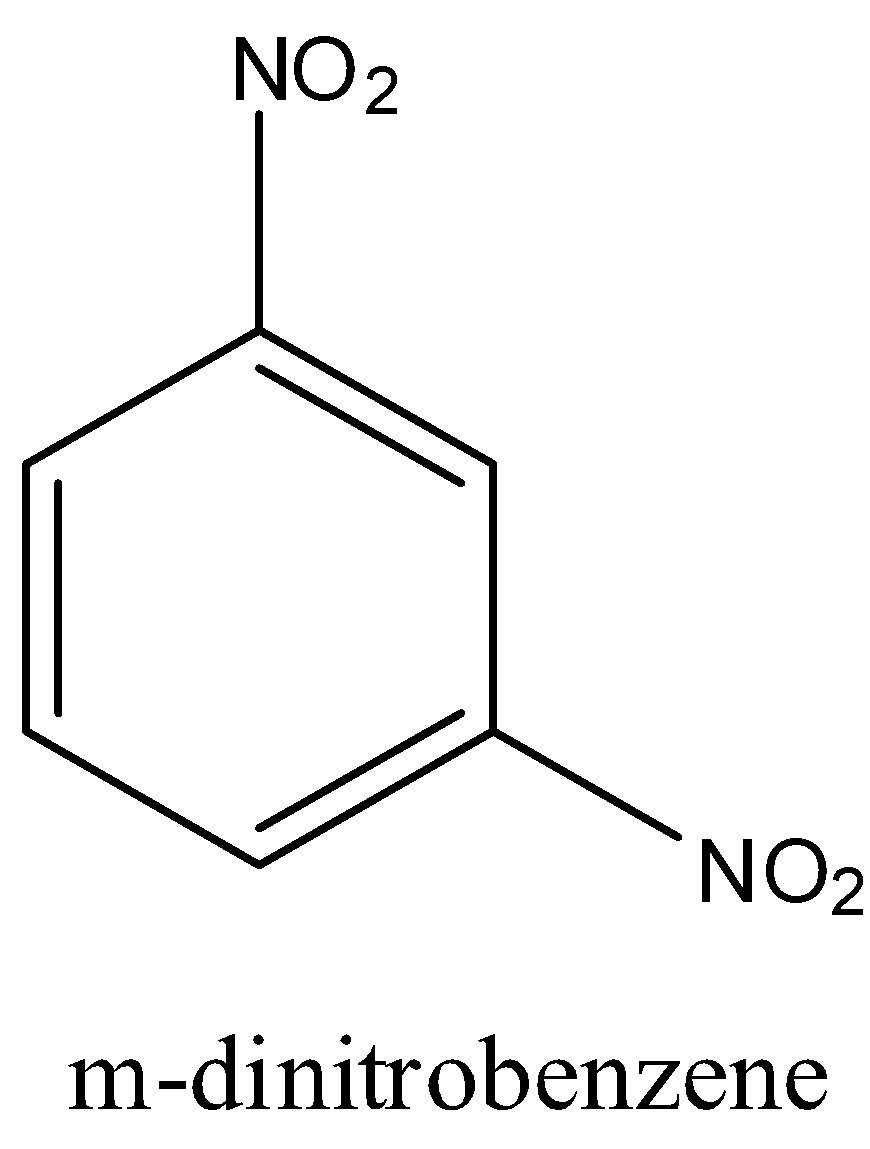
Complete step by step answer:
In the question it is given that, out of benzene, m-dinitrobenzene, and toluene which will undergo nitration most easily.
First we should know the structures of the given compounds.
The structures of the given compounds are as follows.

We know that the ease of nitration depends on the presence of electron density on the given compound to form the respective nitrate derivatives.
We know that Benzene is electron rich in nature but all the electrons are in resonance, so benzene itself won't easily donate electrons or won't easily undergo nitration reaction to form nitro derivative.
Toluene is also called methyl-benzene. Methyl group in toluene makes it an electron donating molecule.
So, ease of nitration is very easy in case of toluene when compared to simple benzene.
Coming to m-dinitrobenzene, the presence of two nitro groups makes the molecule as electron deficient. So, nitration in m-dinitrobenzene is very difficult to do.
Hence, the decreasing order of nitration of the given compounds is as follows:
Toluene > Benzene > m-dinitrobenzene
Note: Don’t be confused with nitro benzene and m-dinitrobenzene.
In nitro benzene only one nitro group will be there but in m-dinitrobenzene there are nitro groups in meta position to each other.
The structure of nitro benzene is

The structure of m-dinitrobenzene is

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




