
Orthoboric acid $ ({H_3}B{O_3}) $ and metaboric acid $ (HB{O_2}) $ differ in respect of
(A) Basicity
(B) Structure
(C) Melting point
(D) None of the above
Answer
546.6k+ views
Hint: To answer the above question we need to state the properties of both the acid such as structures, melting point and basicity. Also we need to discriminate between both the acids on the basis of these three properties.
Complete step by step answer:
METABORIC ACID $ (HB{O_2}) $ : metaboric acid is a common family name for the inorganic compound formed by the dehydration of boric acid. Now we will define metaboric acid on the following three properties:
Basicity: we need $ pK $ value to measure the strength of acid on a logarithmic scale. To find out the $ pK $ value of an acid we need to use the formula;
$ {\mathbf{p}}K = {\log _{10}}(1/{k_a}) $ ;
Where, $ {K_a} $ is the constant used for acid dissociation, now the $ p{K_a} $ value for metaboric acid is 9.236.
Structure: metaboric acid is found in two forms, one is the single molecular one and the other one is the chain polymer one.
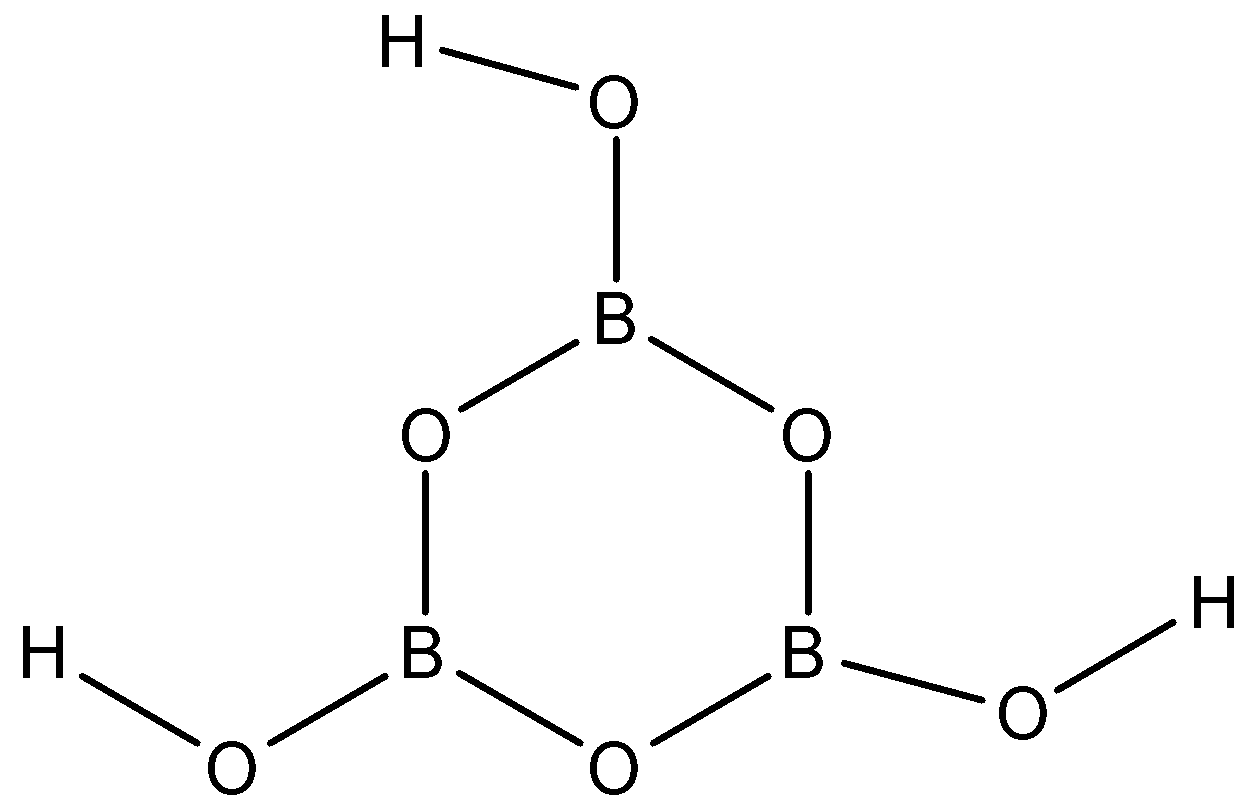
METABORIC ACID
Melting point: the melting point of metaboric acid is $ {236^o}C $
ORTHOBORIC ACID: orthoboric acid is also known as boric acid, hydrogen borate, boracic acid etc. Now we will define orthoboric acid on the following three properties:
-Basicity: the $ p{K_a} $ value for orthoboric acid is 9.24, 12.4, 13.3
-Structure: the molecular formula of orthoboric acid is $ {H_3}B{O_3} $
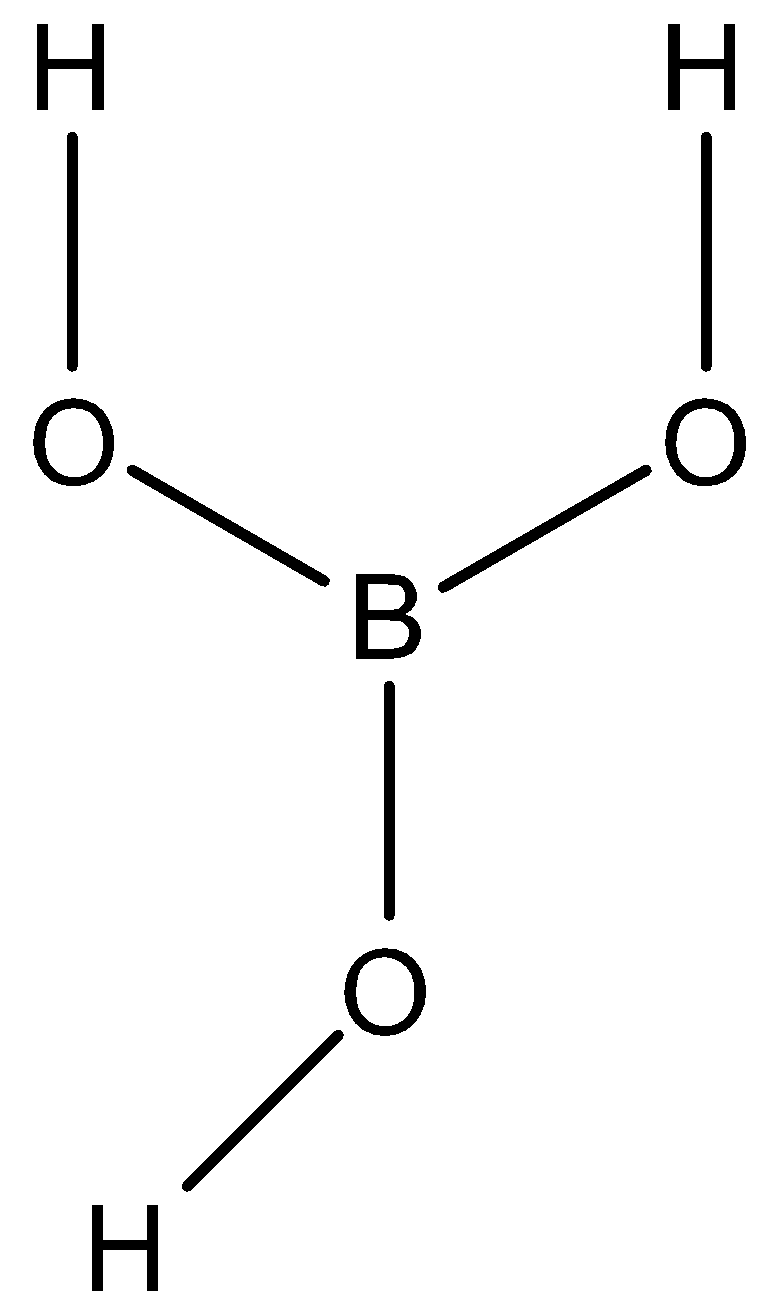
ORTHOBORIC ACID
-Melting point: the melting point of orthoboric acid is $ {170^o}C $
Now according to the above observation of both the acid we can conclude that orthoboric acid and metaboric acid differs on the basis of basicity, structure and melting point. Therefore all the options A, B, C are correct.
Note:
Both the acids majorly differ in their molecular formula and its structure as metaboric acid is present in two forms molecular and chained and orthoboric acid is only present in molecular form in this case the main difference between both acids.
Complete step by step answer:
METABORIC ACID $ (HB{O_2}) $ : metaboric acid is a common family name for the inorganic compound formed by the dehydration of boric acid. Now we will define metaboric acid on the following three properties:
Basicity: we need $ pK $ value to measure the strength of acid on a logarithmic scale. To find out the $ pK $ value of an acid we need to use the formula;
$ {\mathbf{p}}K = {\log _{10}}(1/{k_a}) $ ;
Where, $ {K_a} $ is the constant used for acid dissociation, now the $ p{K_a} $ value for metaboric acid is 9.236.
Structure: metaboric acid is found in two forms, one is the single molecular one and the other one is the chain polymer one.

METABORIC ACID
Melting point: the melting point of metaboric acid is $ {236^o}C $
ORTHOBORIC ACID: orthoboric acid is also known as boric acid, hydrogen borate, boracic acid etc. Now we will define orthoboric acid on the following three properties:
-Basicity: the $ p{K_a} $ value for orthoboric acid is 9.24, 12.4, 13.3
-Structure: the molecular formula of orthoboric acid is $ {H_3}B{O_3} $

ORTHOBORIC ACID
-Melting point: the melting point of orthoboric acid is $ {170^o}C $
Now according to the above observation of both the acid we can conclude that orthoboric acid and metaboric acid differs on the basis of basicity, structure and melting point. Therefore all the options A, B, C are correct.
Note:
Both the acids majorly differ in their molecular formula and its structure as metaboric acid is present in two forms molecular and chained and orthoboric acid is only present in molecular form in this case the main difference between both acids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




