
Orthoboric acid contains:
A.Pyramidal \[B{O_3}^{3 - }\] units
B.Linear \[B{O_3}^{3 - }\] units
C.\[T - \] Shaped \[B{O_3}^{3 - }\]units
D.Triangular \[B{O_3}^{3 - }\] units
Answer
537.3k+ views
Hint: Orthoboric acid is also known as boric acid. It is a weak boron acid sometimes used as an antiseptic, insecticide or neutron absorber and a precursor to other chemical compounds. The simplest borate anion known as the orthoborate ion \[B{O_3}^{3 - }\] is known.
Complete step by step solution: We can predict which \[B{O_3}^{3 - }\] unit contained in orthoboric acid. The structure of orthoboric acid is,
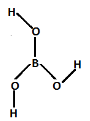
In the structure of orthoboric acid, there are three electron pairs which are three bond pairs. It is \[s{p^2}\] hybridized, the shape of orthoboric acid is triangular planar. The orthoboric acid forms hydrogen bonds with other molecules of orthoboric acid. The orthoboric acid contain Triangular \[B{O_3}^{3 - }\] units
Additional information: Orhtoboric acid has many applications in daily life as well as industry. These are listed below:
Medical use: for minor cut and burn, it can be used as an antiseptic. A very dilute solution of Boric acid can be applied as an eye wash. A dilute solution of boric acid also used as a vaginal douche for the treatment of bacterial vaginosis.
Used as insecticidal: Boric acid can be used as an insecticidal to control ants, cockroaches, fleas and many other insects.
For preserving food: Boric acid is helpful in the preservation of timber against fungal and insect attack.
Used as \[pH\] buffer: Boric acid is widely used as \[pH\] buffer system in equilibrium with its conjugate base-borate ion (mostly in swimming pools).
For lubrication: Boric acid can be used as lubricant on ceramic or metal surfaces. It can also be used to lubricate carrom boards.
Thus, the correct option is D.
Note: The shape of any molecule can be predicted by Valence shell \[{e^ - }\] pair repulsion theory, commonly called as \[VSEPR\] theory. Here \[{e^ - }\] pairs refer to the bond pairs and lone pair \[{e^ - }\]. The geometry of the molecules mainly depends on \[{e^ - }\] pairs.
Complete step by step solution: We can predict which \[B{O_3}^{3 - }\] unit contained in orthoboric acid. The structure of orthoboric acid is,

In the structure of orthoboric acid, there are three electron pairs which are three bond pairs. It is \[s{p^2}\] hybridized, the shape of orthoboric acid is triangular planar. The orthoboric acid forms hydrogen bonds with other molecules of orthoboric acid. The orthoboric acid contain Triangular \[B{O_3}^{3 - }\] units
Additional information: Orhtoboric acid has many applications in daily life as well as industry. These are listed below:
Medical use: for minor cut and burn, it can be used as an antiseptic. A very dilute solution of Boric acid can be applied as an eye wash. A dilute solution of boric acid also used as a vaginal douche for the treatment of bacterial vaginosis.
Used as insecticidal: Boric acid can be used as an insecticidal to control ants, cockroaches, fleas and many other insects.
For preserving food: Boric acid is helpful in the preservation of timber against fungal and insect attack.
Used as \[pH\] buffer: Boric acid is widely used as \[pH\] buffer system in equilibrium with its conjugate base-borate ion (mostly in swimming pools).
For lubrication: Boric acid can be used as lubricant on ceramic or metal surfaces. It can also be used to lubricate carrom boards.
Thus, the correct option is D.
Note: The shape of any molecule can be predicted by Valence shell \[{e^ - }\] pair repulsion theory, commonly called as \[VSEPR\] theory. Here \[{e^ - }\] pairs refer to the bond pairs and lone pair \[{e^ - }\]. The geometry of the molecules mainly depends on \[{e^ - }\] pairs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




