
What is the order of stability for
I) I-butene
II) cis-2-butene
III) trans-2-butene
A) $I$$<$$II$$<$$III$
B) $I$$>$$II$$>$$III$
C) $III$$>$$I$$>$$II$
D) $I$$>$$II$$>$$III$
Answer
568.5k+ views
Hint: Hyperconjugation is one of the phenomena which stabilizes the molecule.
The cis and trans butane are the geometrical isomers of butane.
Complete step by step answer:
Here in the question, the stability order of the given compounds should be compared for getting the answer.
For that we should know a phenomenon called hyperconjugation.
Hyperconjugation is the no bond resonance phenomenon in which the compound stabilizes itself by attracting the electrons from the alpha hydrogen to the nearest carbon.
Alpha hydrogen is the hydrogen of the nearest C with respect to which we speak about the phenomenon.
As the number of alpha hydrogen in the structure is more, more is the hyperconjugation structure and more stable the structure is.
- Now let’s consider the options and comment on its stability.
The options given here is - I-butene, cis-2-butene and trans-2-butene
Cis and trans butane are the isomers of butene.
Consider the structures of the three compounds.
First let’s see the structure of 1-butene,
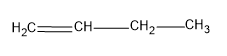
It has only two alpha hydrogens, so only two hyperconjugations are possible for this structure. Hence the stability of this structure will be low.
Now let’s consider the structure of cis-2-butene.
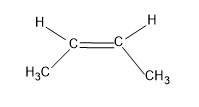
Here there are six alpha hydrogens which are attached to the carbons but the methyl group is present in the same side which causes steric hindrance.And steric hindrance decreases the stability of the compound.
The last option is trans-2-butene
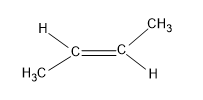
Here also six alpha hydrogens are present but the methyl group is present on the opposite side of the carbon, hence it stabilizes the structure.
So we can conclude that, as in 1-butene there is only two alpha hydrogen present, it will be the least stable structure. And comparing cis and trans isomers, cis will have the least stability since there is steric hindrance in the structure and trans-2-butene will be the most stable structure among the given options,
Hence the correct option is option (A). $I$$<$$II$$<$$III$
Note: The more substituted alkene will be the most stable one than the least substituted alkenes. And the least substituted alkenes are more stable than the unsubstituted or normal alkene.
Here the order of stability is, Alkene $<$ substituted alkenes.
Steric effect is another one factor that should be given consideration, while comparing the stabilities of the structure.
The cis and trans butane are the geometrical isomers of butane.
Complete step by step answer:
Here in the question, the stability order of the given compounds should be compared for getting the answer.
For that we should know a phenomenon called hyperconjugation.
Hyperconjugation is the no bond resonance phenomenon in which the compound stabilizes itself by attracting the electrons from the alpha hydrogen to the nearest carbon.
Alpha hydrogen is the hydrogen of the nearest C with respect to which we speak about the phenomenon.
As the number of alpha hydrogen in the structure is more, more is the hyperconjugation structure and more stable the structure is.
- Now let’s consider the options and comment on its stability.
The options given here is - I-butene, cis-2-butene and trans-2-butene
Cis and trans butane are the isomers of butene.
Consider the structures of the three compounds.
First let’s see the structure of 1-butene,

It has only two alpha hydrogens, so only two hyperconjugations are possible for this structure. Hence the stability of this structure will be low.
Now let’s consider the structure of cis-2-butene.

Here there are six alpha hydrogens which are attached to the carbons but the methyl group is present in the same side which causes steric hindrance.And steric hindrance decreases the stability of the compound.
The last option is trans-2-butene

Here also six alpha hydrogens are present but the methyl group is present on the opposite side of the carbon, hence it stabilizes the structure.
So we can conclude that, as in 1-butene there is only two alpha hydrogen present, it will be the least stable structure. And comparing cis and trans isomers, cis will have the least stability since there is steric hindrance in the structure and trans-2-butene will be the most stable structure among the given options,
Hence the correct option is option (A). $I$$<$$II$$<$$III$
Note: The more substituted alkene will be the most stable one than the least substituted alkenes. And the least substituted alkenes are more stable than the unsubstituted or normal alkene.
Here the order of stability is, Alkene $<$ substituted alkenes.
Steric effect is another one factor that should be given consideration, while comparing the stabilities of the structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




