
How many optically active stereoisomers are possible for butane-2, 3-diol.
(A) 1
(B) 2
(C) 3
(D) 4
Answer
544.2k+ views
Hint: In order to solve this question we need to have to the study stereoisomerism then will have to see all the possible structure of the butan-2, 3-diol then will study the stereoisomerism of the bautan-2, 3 diol.
Complete step by step solution:
For solving this the stereoisomerism so:
Generally defined, stereoisomers are isomers that have the same composition (that is, the same parts) but that differ in the orientation of those parts in space. There are two kinds of stereoisomers: enantiomers and diastereomers.
Now we will learning it one by one:
Enantiomers are a pair of molecules that exist in two forms that are mirror images of one another but cannot be superimposed one upon the other.
Diastereomers are defined as compounds which have the same molecular formula and sequence of bonded elements but which are non-superimposable, non-mirror images.
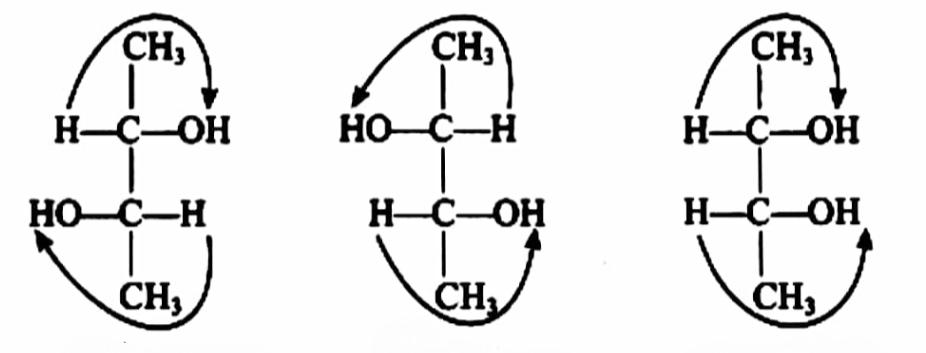
The number of optically active stereoisomers possible for 2,3−diol is 2. They are d,l isomers which are optically active. The meso- compound is optically inactive due to internal compensation.
So we can say that the correct option is B.
Note:
2,3-Butanediol is the organic compound with the formula $ {(C{H_3}CHOH)_2} $ . It is classified as a vic-diol (glycol). It exists as three stereoisomers, a chiral pair and the meso isomer. All are colorless liquids.
Complete step by step solution:
For solving this the stereoisomerism so:
Generally defined, stereoisomers are isomers that have the same composition (that is, the same parts) but that differ in the orientation of those parts in space. There are two kinds of stereoisomers: enantiomers and diastereomers.
Now we will learning it one by one:
Enantiomers are a pair of molecules that exist in two forms that are mirror images of one another but cannot be superimposed one upon the other.
Diastereomers are defined as compounds which have the same molecular formula and sequence of bonded elements but which are non-superimposable, non-mirror images.

The number of optically active stereoisomers possible for 2,3−diol is 2. They are d,l isomers which are optically active. The meso- compound is optically inactive due to internal compensation.
So we can say that the correct option is B.
Note:
2,3-Butanediol is the organic compound with the formula $ {(C{H_3}CHOH)_2} $ . It is classified as a vic-diol (glycol). It exists as three stereoisomers, a chiral pair and the meso isomer. All are colorless liquids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




