
How many optical isomers does isoleucine have?
Answer
551.4k+ views
Hint: In terms of molecular weight as well as chemical and physical properties, optical isomerism is a case where the isomers display identical characteristics. They differ in their impact on the rotation of polarized light, however. $ {C_6}{H_{13}}N{O_2} $ is the chemical formula of isoleucine.
Formula Used
We will use the following expression to find the number of optical isomers
$ {2^n} $
Where, $ n $ is the number of chiral centres of the chemical compound.
Complete Step-by-Step Solution
Optical isomerism mainly occurs in substances with the same structural and molecular formula, but cannot be superimposed on each other. We can say, in simple words, that they are mirror images of one another. Alternatively, it can also be found in substances that have an asymmetric carbon atom.
The IUPAC name for isoleucine is $ 2 - $ amino $ 3 - $ methylpentanoic acid.
$ {C_6}{H_{13}}N{O_2} $ is the chemical formula of isoleucine. It has a molecular weight of $ 131.17 {\text{g/mol}} $ .
Let us draw the structure of isoleucine and observe its various properties.
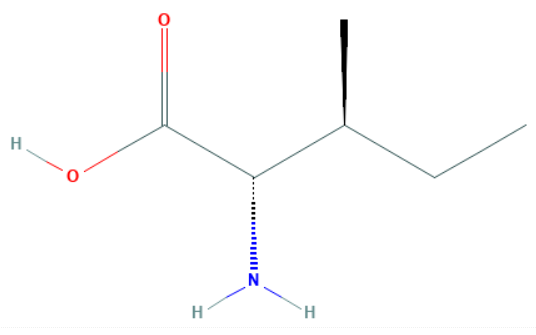
We can observe that there are a total of two chiral centres in isoleucine at the second and third carbon atoms.
Since, there are two chiral centres, we will use our formula to find out the number of optical isomers.
That is
$ {2^n} $
Let us substitute the number of chiral centres in the above formula to get our result
$ {2^2} = 4 $
Hence, there are a total of $ 4 $ optical isomers of isoleucine.
Note
The first step is to find the chiral centres of the molecule in order to come to a solution. It is possible to explain the difference between chiral and achiral molecules on the basis of the symmetry plane. If the entire group attached to the central carbon atom is different, then there is no symmetry plane. A molecule like this is known as a chiral molecule.
Formula Used
We will use the following expression to find the number of optical isomers
$ {2^n} $
Where, $ n $ is the number of chiral centres of the chemical compound.
Complete Step-by-Step Solution
Optical isomerism mainly occurs in substances with the same structural and molecular formula, but cannot be superimposed on each other. We can say, in simple words, that they are mirror images of one another. Alternatively, it can also be found in substances that have an asymmetric carbon atom.
The IUPAC name for isoleucine is $ 2 - $ amino $ 3 - $ methylpentanoic acid.
$ {C_6}{H_{13}}N{O_2} $ is the chemical formula of isoleucine. It has a molecular weight of $ 131.17 {\text{g/mol}} $ .
Let us draw the structure of isoleucine and observe its various properties.

We can observe that there are a total of two chiral centres in isoleucine at the second and third carbon atoms.
Since, there are two chiral centres, we will use our formula to find out the number of optical isomers.
That is
$ {2^n} $
Let us substitute the number of chiral centres in the above formula to get our result
$ {2^2} = 4 $
Hence, there are a total of $ 4 $ optical isomers of isoleucine.
Note
The first step is to find the chiral centres of the molecule in order to come to a solution. It is possible to explain the difference between chiral and achiral molecules on the basis of the symmetry plane. If the entire group attached to the central carbon atom is different, then there is no symmetry plane. A molecule like this is known as a chiral molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




