
Optical isomerism is shown by:
a.) Oxalic acid
b.) Benzoic acid
c.) Acetic acid
d.) Lactic acid
Answer
601.5k+ views
Hint: Optical isomers are molecules that differ from each other in their behavior towards plane-polarized light. They have different three-dimensional arrangements of the same atoms/groups in a molecule.
Complete step-by-step answer:
Chiral carbon: The carbon atom which has four different groups attached to it are called chiral carbons.
A chiral molecule is non-superimposable on its mirror image.
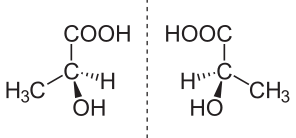
The following are the structures of given compounds;
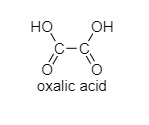
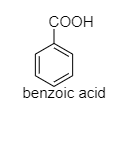
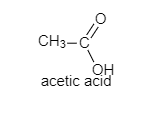
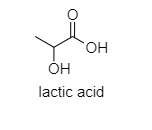
As we can see here that oxalic acid, benzoic acid, and acetic acid are achiral. It means, they do not have four different groups attached to the carbon while Lactic acid contains a chiral carbon as it has four different groups attached to it. In lactic acid, there is one asymmetric carbon atom. It also does not contain any element of symmetry.
Symmetry elements:- It is a line, point, or plane through an object about which rotation or reflection occurs.
Hence, lactic acid is optically active while others are optically inactive.
Additional information:
Enantiomers: It is defined as the stereoisomers of a compound which are related to each other as non-superimposable mirror images. They possess identical physical properties like melting point, boiling point solubility, refractive index, etc.
One of the enantiomers rotates the plane of polarization to the left is called laevorotatory while the other one rotates the plane of polarization to the right is called dextrorotatory.
Thus, the number of enantiomers in lactic acid = ${ 2 }^{ n }{ =2 }^{ 1 }$
Where n = number of the chiral carbon
So, there are two enantiomers of lactic acid.
Note: The possibility for the mistake is that you can choose option A. But in oxalic acid, two carbonyl groups are present which make them achiral.
Complete step-by-step answer:
Chiral carbon: The carbon atom which has four different groups attached to it are called chiral carbons.
A chiral molecule is non-superimposable on its mirror image.
The following are the structures of given compounds;




As we can see here that oxalic acid, benzoic acid, and acetic acid are achiral. It means, they do not have four different groups attached to the carbon while Lactic acid contains a chiral carbon as it has four different groups attached to it. In lactic acid, there is one asymmetric carbon atom. It also does not contain any element of symmetry.
Symmetry elements:- It is a line, point, or plane through an object about which rotation or reflection occurs.
Hence, lactic acid is optically active while others are optically inactive.
Additional information:
Enantiomers: It is defined as the stereoisomers of a compound which are related to each other as non-superimposable mirror images. They possess identical physical properties like melting point, boiling point solubility, refractive index, etc.
One of the enantiomers rotates the plane of polarization to the left is called laevorotatory while the other one rotates the plane of polarization to the right is called dextrorotatory.
Thus, the number of enantiomers in lactic acid = ${ 2 }^{ n }{ =2 }^{ 1 }$
Where n = number of the chiral carbon
So, there are two enantiomers of lactic acid.

Note: The possibility for the mistake is that you can choose option A. But in oxalic acid, two carbonyl groups are present which make them achiral.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




