
One of the resonating structure of \[{\text{S}}{{\text{O}}_{\text{4}}}^{{\text{ - 2}}}\] is as shown.
Which set formal charge on oxygen and bond order is correct?
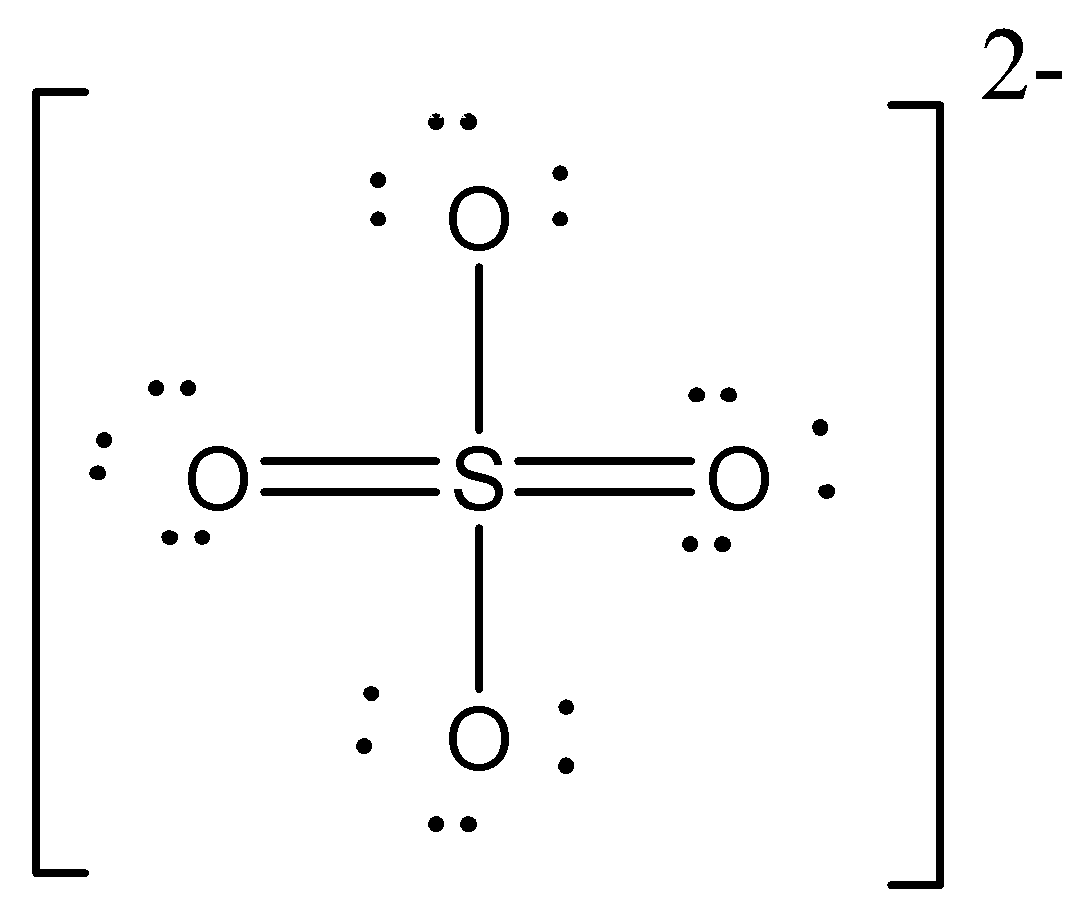
A. \[ - 0.5\] and \[1.5\]
B. \[1.5\] and \[3\]
C. \[2\] and \[3\]
D. \[1.5\] and \[1.5\]

Answer
577.8k+ views
Hint: Resonating Structures are different structures which shows the possibility of how the bonds may exist in several combinations. \[{\text{S}}{{\text{O}}_{\text{4}}}^{{\text{ - 2}}}\], which is having a total of \[6\] resonating structure.
Complete step by step answer:
Let’s start with discussing the resonating structures, Resonating Structures are different structures which shows the possibility of how the bonds may exist in several combinations. Since we can’t see the bonds themselves, we can always make some assumptions on how the bonds might exist. More the resonating structures are stronger the stronger the two molecules are bonded. Resonance structures are also called resonance hybrids in valence bond theory.
We are given with \[{\text{S}}{{\text{O}}_{\text{4}}}^{{\text{ - 2}}}\], which is having a total of 6 resonating structure. The resonating structures are given below:
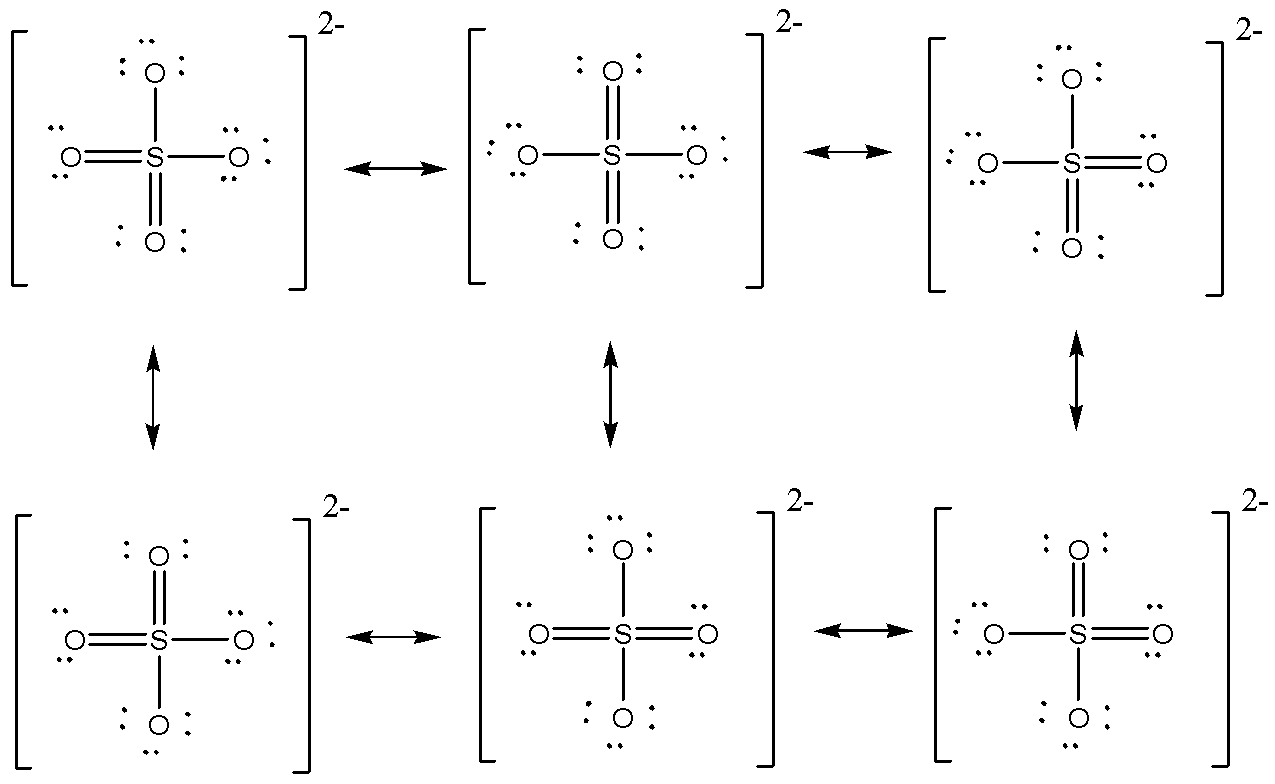
So let’s check the formal charge on first (1), which will be equal to $\dfrac{{{\text{Sum of charges on all six structures}}}}{6} = \dfrac{{ - 1 + 0 - 1 + 0 - 1 + 0}}{6} = - 0.5$,
Hence formal charge on 1st is \[ - 0.5\].
Let’s check for bond order, which will be equal to
$\dfrac{{1 + 2 + 1 + 2 + 1 + 2}}{6} = \dfrac{3}{2} = 1.5$
Hence the answer to this question is option A. \[ - 0.5\] and \[1.5\].
So, the correct answer is Option A .
Note:
\[{\text{S}}{{\text{O}}_{\text{4}}}^{{\text{ - 2}}}\] ion popularly known as sulphate is present in compounds like sulphuric acid and in many other such compounds. Sulphuric acid with chemical formula \[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\] is one of the most acidic acid and is used in various industries. This chemical is used in manufacturing of fertilizers, pigments, drugs, explosives, etc.
Complete step by step answer:
Let’s start with discussing the resonating structures, Resonating Structures are different structures which shows the possibility of how the bonds may exist in several combinations. Since we can’t see the bonds themselves, we can always make some assumptions on how the bonds might exist. More the resonating structures are stronger the stronger the two molecules are bonded. Resonance structures are also called resonance hybrids in valence bond theory.
We are given with \[{\text{S}}{{\text{O}}_{\text{4}}}^{{\text{ - 2}}}\], which is having a total of 6 resonating structure. The resonating structures are given below:

So let’s check the formal charge on first (1), which will be equal to $\dfrac{{{\text{Sum of charges on all six structures}}}}{6} = \dfrac{{ - 1 + 0 - 1 + 0 - 1 + 0}}{6} = - 0.5$,
Hence formal charge on 1st is \[ - 0.5\].
Let’s check for bond order, which will be equal to
$\dfrac{{1 + 2 + 1 + 2 + 1 + 2}}{6} = \dfrac{3}{2} = 1.5$
Hence the answer to this question is option A. \[ - 0.5\] and \[1.5\].
So, the correct answer is Option A .
Note:
\[{\text{S}}{{\text{O}}_{\text{4}}}^{{\text{ - 2}}}\] ion popularly known as sulphate is present in compounds like sulphuric acid and in many other such compounds. Sulphuric acid with chemical formula \[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\] is one of the most acidic acid and is used in various industries. This chemical is used in manufacturing of fertilizers, pigments, drugs, explosives, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




