
One mole of an ideal diatomic gas is taken through the cycle as shown in the figure:
\[1 \to 2\]: isochoric process
\[2 \to 3\]: a straight line of P-V diagram
\[3 \to 1\]: isobaric process
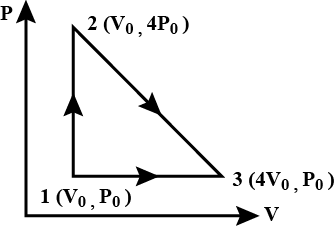
The average molecular speed of the gas in the states 1, 2 and 3 in the ratio:
A. \[1:2:2\]
B. \[1:\sqrt 2 :\sqrt 2 \]
C. \[1:1:1\]
D. \[1:2:4\]

Answer
574.2k+ views
Hint: In \[1 \to 2\], Pressure is directly proportional to temperature. In \[3 \to 1\], Volume is directly proportional to Temperature. And the average molecular speed \[\left( {{V_{rms}}} \right)\] is directly proportional to $\sqrt {\dfrac{{RT}}{M}} $, hence \[v \propto \sqrt T \]
Complete step by step answer:
In the state 1
Let temperature be \[{T_o}\]
and we know that, \[PV = nRT\]
In the state 2
Since \[1 \to 2\] is an isochoric process
Hence, in State 1
$\dfrac{P}{T} = $ Constant
\[v \propto {T_0}\]
when Pressure is equal to \[4{P_o}\]. Thus, the Temperature \[ = 4{T_o}\]
State 3
Since \[3 \to 1\] is an isobaric process,
According to Charlee’s law
\[V \propto T\]
$\dfrac{V}{T} = $ Constant
When Volume is \[4{V_o}\]. Thus Temperature \[ = 4{T_o}\]
The root-mean-square speed or the average molecular speed \[\left( {{V_{rms}}} \right)\] is directly proportional to $\sqrt {\dfrac{{RT}}{M}} $
\[ \Rightarrow v \propto \sqrt T \]
The average velocity of the gas molecule has the formula : ${V_{avg}} = \sqrt {\dfrac{{8RT}}{M}} $
Where,
V= molecular speed of the particle
T = Temperature in Kelvin
M = molar mass of the compound
R = Ideal gas constant
Hence,
State 1, \[v \propto {T_0}\]
State 2, \[v \propto \sqrt {4{T_o}} = 2\sqrt {{T_o}} \]
State3, \[v \propto \sqrt {4{T_o}} = 2\sqrt {{T_o}} \]
Hence, \[{V_1}:{V_2}:{V_3} = \sqrt {{T_o}} :\sqrt {4{T_o}} :\sqrt {4Y{T_o}} \]
Ratio \[ = 1:2:2\]
Therefore, the correct answer is option (A).
Note: Isobaric process is carried out at a constant pressure. In such a process \[dP = 0\]. Isochoric process is a process in which the volume of the system remains constant, whereby \[dV = 0\]. According to the Kinetic Molecular Theory of Gases, the molecular speed of the gas explains that gas particles are in continuous motion and they exhibit ideally elastic collisions.
Complete step by step answer:
In the state 1
Let temperature be \[{T_o}\]
and we know that, \[PV = nRT\]
In the state 2
Since \[1 \to 2\] is an isochoric process
Hence, in State 1
$\dfrac{P}{T} = $ Constant
\[v \propto {T_0}\]
when Pressure is equal to \[4{P_o}\]. Thus, the Temperature \[ = 4{T_o}\]
State 3
Since \[3 \to 1\] is an isobaric process,
According to Charlee’s law
\[V \propto T\]
$\dfrac{V}{T} = $ Constant
When Volume is \[4{V_o}\]. Thus Temperature \[ = 4{T_o}\]
The root-mean-square speed or the average molecular speed \[\left( {{V_{rms}}} \right)\] is directly proportional to $\sqrt {\dfrac{{RT}}{M}} $
\[ \Rightarrow v \propto \sqrt T \]
The average velocity of the gas molecule has the formula : ${V_{avg}} = \sqrt {\dfrac{{8RT}}{M}} $
Where,
V= molecular speed of the particle
T = Temperature in Kelvin
M = molar mass of the compound
R = Ideal gas constant
Hence,
State 1, \[v \propto {T_0}\]
State 2, \[v \propto \sqrt {4{T_o}} = 2\sqrt {{T_o}} \]
State3, \[v \propto \sqrt {4{T_o}} = 2\sqrt {{T_o}} \]
Hence, \[{V_1}:{V_2}:{V_3} = \sqrt {{T_o}} :\sqrt {4{T_o}} :\sqrt {4Y{T_o}} \]
Ratio \[ = 1:2:2\]
Therefore, the correct answer is option (A).
Note: Isobaric process is carried out at a constant pressure. In such a process \[dP = 0\]. Isochoric process is a process in which the volume of the system remains constant, whereby \[dV = 0\]. According to the Kinetic Molecular Theory of Gases, the molecular speed of the gas explains that gas particles are in continuous motion and they exhibit ideally elastic collisions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




