
On the periodic table, how is atomic mass represented?
Answer
550.5k+ views
Hint: Periodic table is known as the tabular arrangement of the chemical elements which are arranged on the basis of atomic number. In the periodic table there are vertical columns which are known as groups and the horizontal rows are known as periods. The properties of the elements are the periodic functions of their atomic numbers.
Complete step by step answer:
The atomic mass is defined as the mass of the one atom present in that element. We can measure it with help of mass spectrometry. The atomic mass is measured in terms of atomic mass unit ( amu ). The atomic mass unit is defined as the mass which is equal to the $$\dfrac{1}{{12}}$$ of the mass of the single atom of the carbon 12 atom. In the periodic table the elements are characterised by their atomic number represented as Z. Beneath the symbol of the element the atomic mass of that element is present which is also generally the weighted average of the element's particular isotopic distribution. Now let us take the example of element calcium whose symbol is Ca and have the atomic number 20 and atomic mass 40.08. so it is represented in the periodic table as the following:
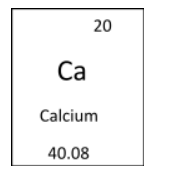
Ca
Atomic Weight = 40.078
Atomic number = 20
Note: There are eighteen groups and seven periods in the periodic table. The atomic size increases from left to right in the period and ionisation energy and electron affinity also increases from left to right in the period. In the group the atomic size increases and electron affinity and ionisation energy decreases down the group.
Complete step by step answer:
The atomic mass is defined as the mass of the one atom present in that element. We can measure it with help of mass spectrometry. The atomic mass is measured in terms of atomic mass unit ( amu ). The atomic mass unit is defined as the mass which is equal to the $$\dfrac{1}{{12}}$$ of the mass of the single atom of the carbon 12 atom. In the periodic table the elements are characterised by their atomic number represented as Z. Beneath the symbol of the element the atomic mass of that element is present which is also generally the weighted average of the element's particular isotopic distribution. Now let us take the example of element calcium whose symbol is Ca and have the atomic number 20 and atomic mass 40.08. so it is represented in the periodic table as the following:

Ca
Atomic Weight = 40.078
Atomic number = 20
Note: There are eighteen groups and seven periods in the periodic table. The atomic size increases from left to right in the period and ionisation energy and electron affinity also increases from left to right in the period. In the group the atomic size increases and electron affinity and ionisation energy decreases down the group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




