
Of the following molecules, the ones which are hypervalent in their dimeric form:
$ Cl{O_3}\,,\,OF\,,\,Ga{H_3}\,,\,AlC{l_3}\,,\,IC{l_3}\,,\,N{O_2},\,Co{(CO)_4} $
(A) $ Cl{O_3}\,,\,Ga{H_3},\,IC{l_3} $
(B) $ Cl{O_3}\,,\,\,IC{l_3}\,,\,Co{(CO)_4} $
(C) $ OF\,,N{O_2},Ga{H_3} $
(D) $ Ga{H_3}\,,Cl{O_3}\,,\,IC{l_3}\,,\,Co{(CO)_4} $
Answer
509.4k+ views
Hint :Hypervalent, the name itself indicates that there are more electrons in the valence shell of the molecules. This phenomenon is also termed as expanded octet. A dimer is formed when two monomers are joined by a bond.
Complete Step By Step Answer:
We know that the outermost electron shell of an atom which contains valence electrons is termed as valence shell. These electrons participate in the chemical bond formation. These valence electrons help in determining the chemical properties of an element. In the case of the main group element, valence electrons exist only in the outermost electron shell whereas in case of transition metal, a valence electron can also exist in the inner shell.
A hypervalent molecule which contains one or more main group elements that contain more than eight electrons in the valence shell of the main group elements. There are some specific classes of hypervalent molecules such as noble gas compounds like $ Xe{F_4} $ , tetra-, penta- and hexavalent phosphorous, silicon and sulfur compounds like $ PC{l_5}\,,S{F_6} $ and hypervalent iodine like $ \,I{F_7} $ etc.
Now, we have to predict the hypervalent molecules in dimeric form. So, first we should know about the dimeric form. A dimeric form is an oligomer which is formed by joining two monomers by a bond. This bond may be covalent or intermolecular, strong or weak. When the two monomers are same then it is known as homodimer whereas when the two monomers are different then, it is known as heterodimer.
Make structures of the dimers of the molecules to know whether it is hypervalent or not. In the given question, there are only three dimers of molecules which exist as hypervalent..
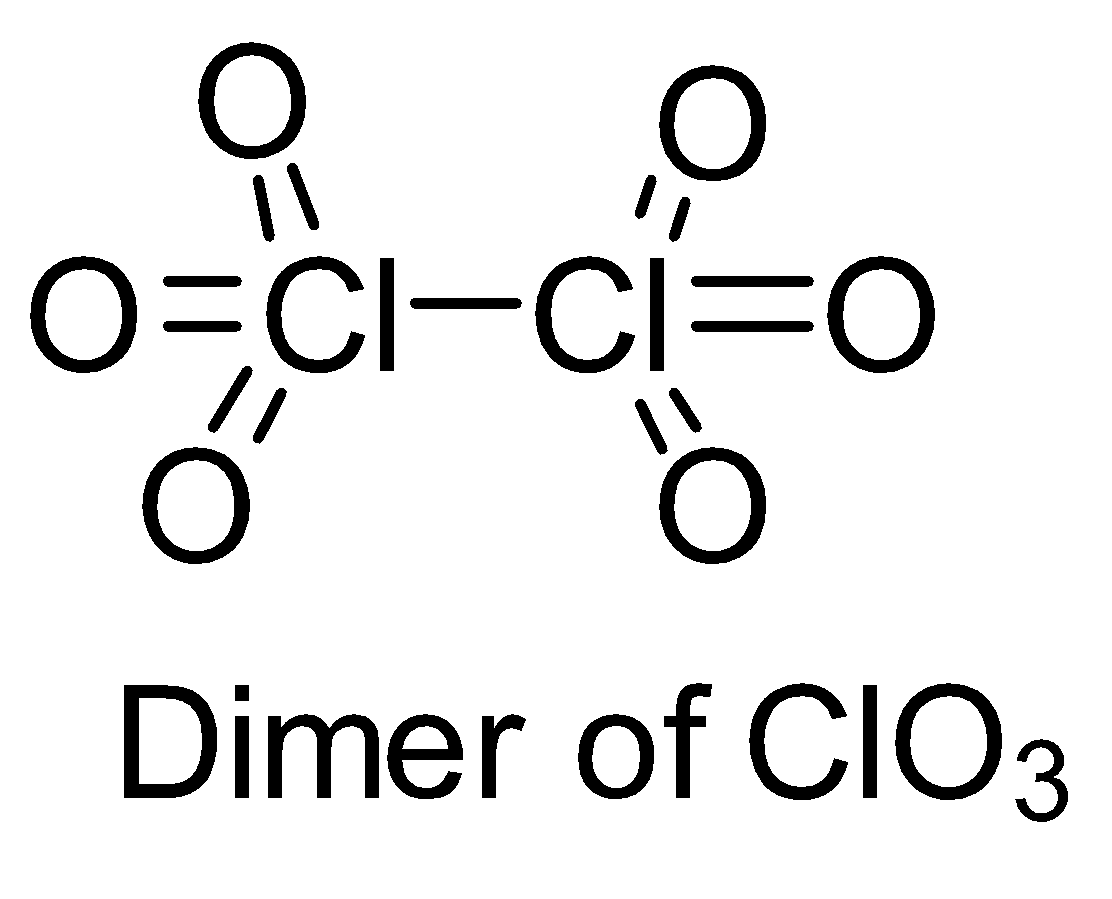
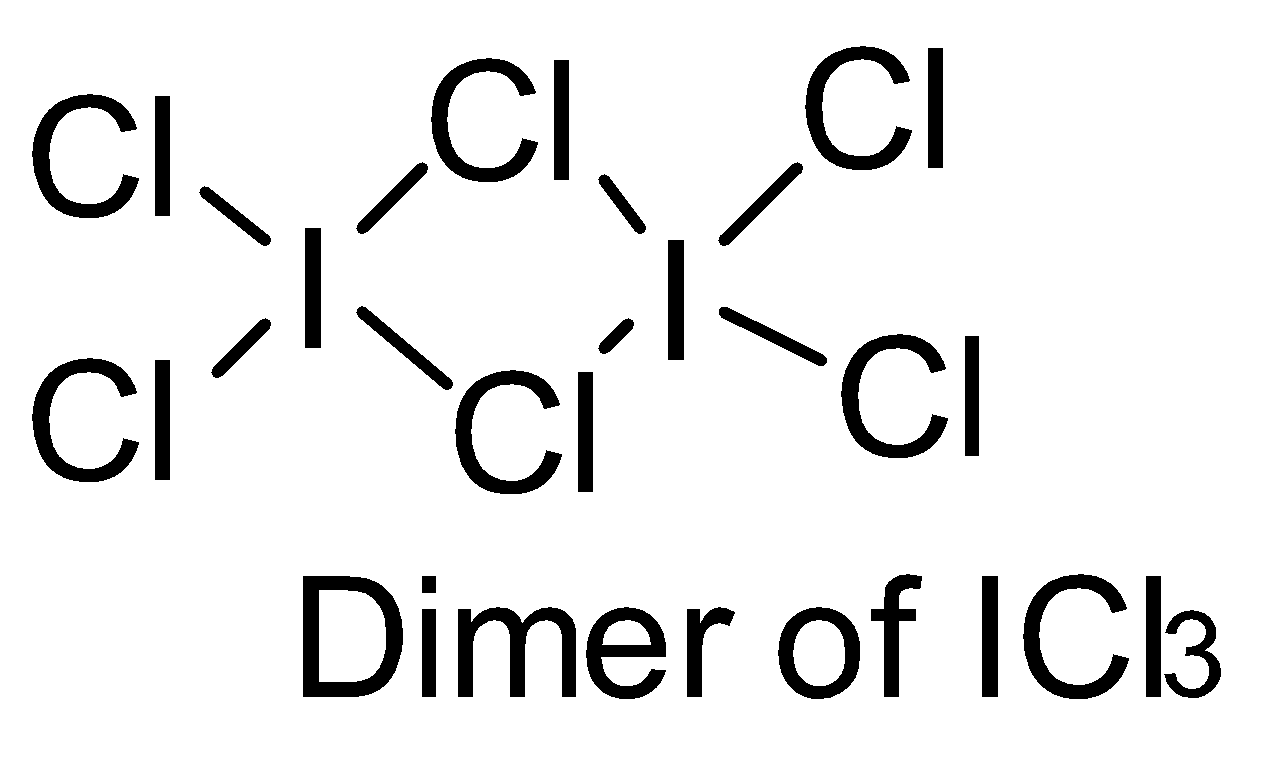
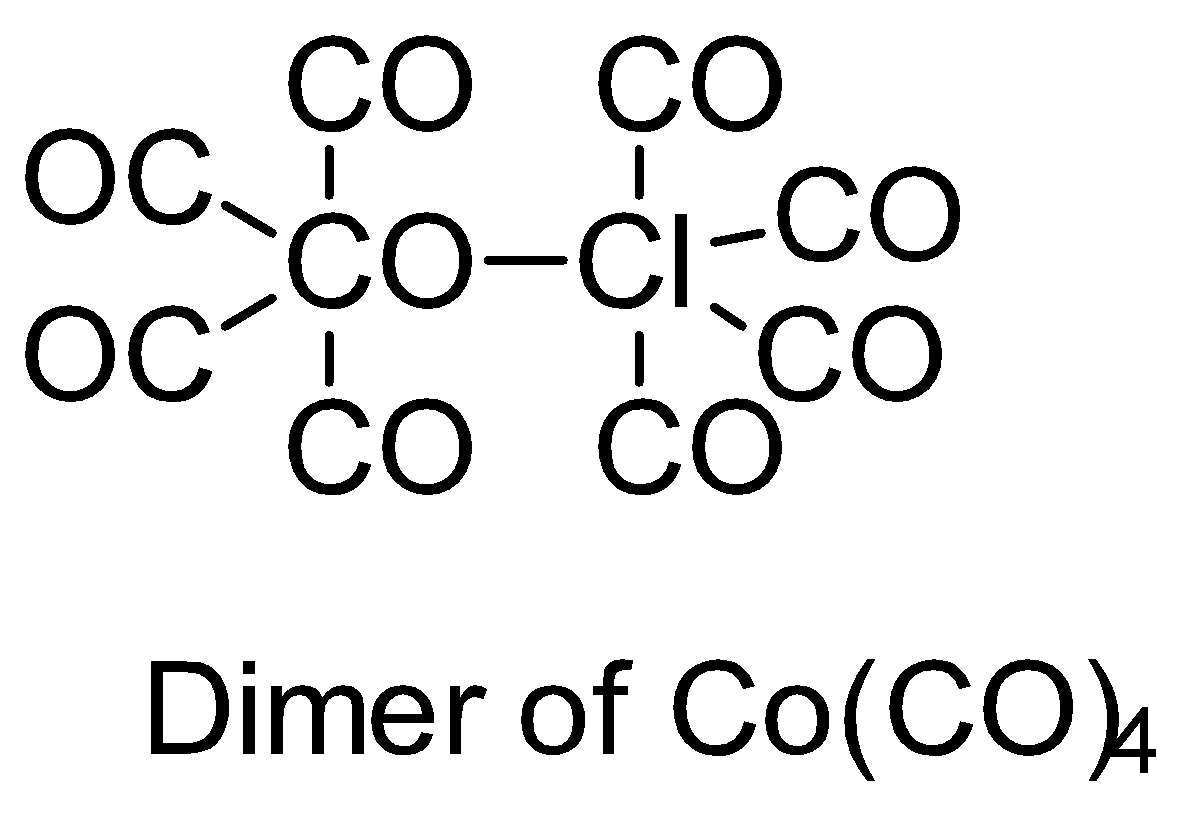
Hence, the correct answer is option (B).
Note :
Hypervalency is a concept based on hybrid orbital theory and Lewis theory. It is useful in predicting the molecular shape and how atoms are connected to each other. Dimerization occurs when two molecules dimerise by a bond and the reverse of dimerization is called dissociation.
Complete Step By Step Answer:
We know that the outermost electron shell of an atom which contains valence electrons is termed as valence shell. These electrons participate in the chemical bond formation. These valence electrons help in determining the chemical properties of an element. In the case of the main group element, valence electrons exist only in the outermost electron shell whereas in case of transition metal, a valence electron can also exist in the inner shell.
A hypervalent molecule which contains one or more main group elements that contain more than eight electrons in the valence shell of the main group elements. There are some specific classes of hypervalent molecules such as noble gas compounds like $ Xe{F_4} $ , tetra-, penta- and hexavalent phosphorous, silicon and sulfur compounds like $ PC{l_5}\,,S{F_6} $ and hypervalent iodine like $ \,I{F_7} $ etc.
Now, we have to predict the hypervalent molecules in dimeric form. So, first we should know about the dimeric form. A dimeric form is an oligomer which is formed by joining two monomers by a bond. This bond may be covalent or intermolecular, strong or weak. When the two monomers are same then it is known as homodimer whereas when the two monomers are different then, it is known as heterodimer.
Make structures of the dimers of the molecules to know whether it is hypervalent or not. In the given question, there are only three dimers of molecules which exist as hypervalent..

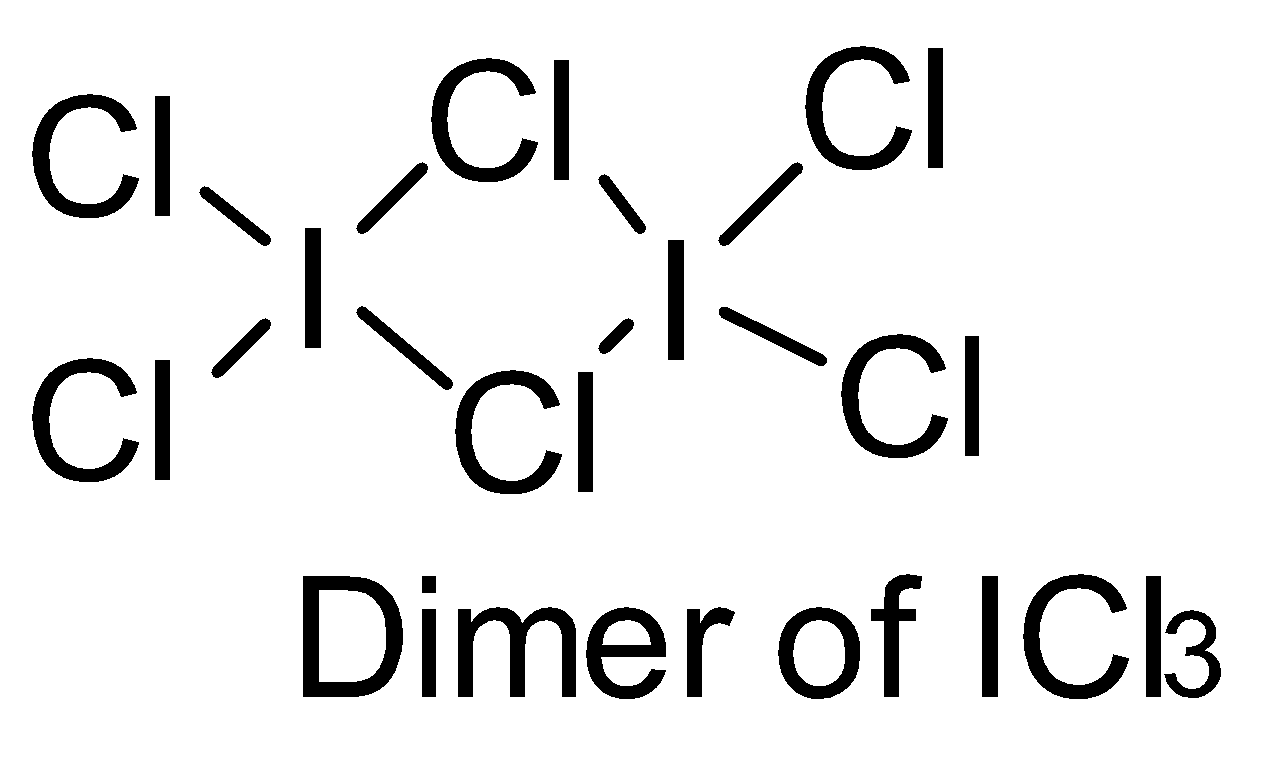
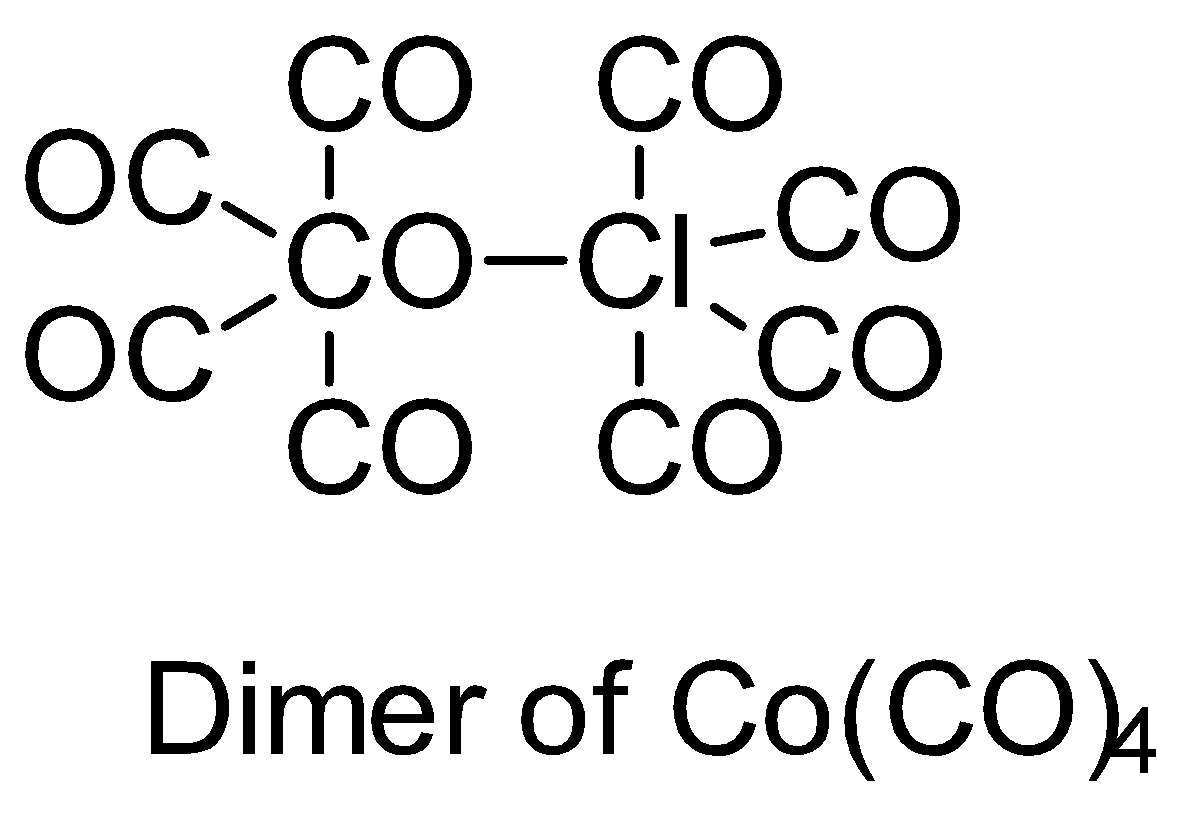
Hence, the correct answer is option (B).
Note :
Hypervalency is a concept based on hybrid orbital theory and Lewis theory. It is useful in predicting the molecular shape and how atoms are connected to each other. Dimerization occurs when two molecules dimerise by a bond and the reverse of dimerization is called dissociation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




