
Octet rule is not followed in:
A. $CC{l_4}\,,\,{N_2}{O_4}\,and\,{N_2}{O_5}$
B. $B{F_3}\,,\,BeC{l_2}\,and\,N{O_2}$
C. $NaCl\,,\,MgC{l_2}\,,\,MgO$
D. $PC{l_3}\,,\,N{H_3}\,,\,{H_2}O$
Answer
533.7k+ views
Hint: In order to answer this question, you must recall the concept of Octet Rule. Just go through the Rule and decide which among the following options is following the Octet rule, and you will get your required answer. And select the correct option.
Complete step by step answer:
The octet rule is a chemical rule of thumb that reflects the observation that main group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium or magnesium. The valence electrons can be counted using a Lewis electron dot diagram.
Step 2: From the above given options:
Octet rule is not followed in: $B{F_3}$ $ \Rightarrow $ Boron utilizes 3 of its electrons for pairing, receiving 1 electron each from F, the outermost shell of B is incomplete with 6 electrons.
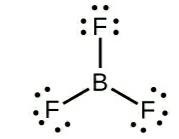
$BeC{l_2}\, \Rightarrow $ has 2 electrons in the outermost shell, that get involved in pairing, the outermost shell of Be is incomplete with 4 electrons.
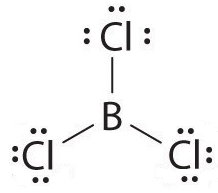
$N{O_2}\, \Rightarrow \,N$ has 5 valence electrons, it enters into a double bond with one O atom, and donates 1 lone pair to another, the outermost shell has 5 electrons.
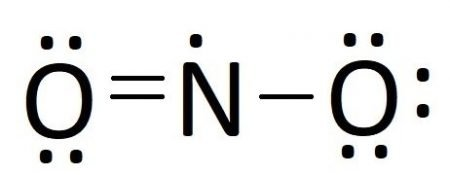
So, the correct answer is Option B.
Note: Some main group elements have the ability to form hypervalent compounds. Examples include sulfur hexafluoride and phosphorus pentachloride. If all the phosphorus-chlorine bonds in a molecule are covalent, it would imply that the phosphorus molecule is violating the octet rule by holding a total of 10 valence electrons.
Complete step by step answer:
The octet rule is a chemical rule of thumb that reflects the observation that main group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium or magnesium. The valence electrons can be counted using a Lewis electron dot diagram.
Step 2: From the above given options:
Octet rule is not followed in: $B{F_3}$ $ \Rightarrow $ Boron utilizes 3 of its electrons for pairing, receiving 1 electron each from F, the outermost shell of B is incomplete with 6 electrons.

$BeC{l_2}\, \Rightarrow $ has 2 electrons in the outermost shell, that get involved in pairing, the outermost shell of Be is incomplete with 4 electrons.

$N{O_2}\, \Rightarrow \,N$ has 5 valence electrons, it enters into a double bond with one O atom, and donates 1 lone pair to another, the outermost shell has 5 electrons.
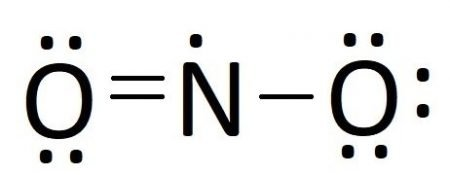
So, the correct answer is Option B.
Note: Some main group elements have the ability to form hypervalent compounds. Examples include sulfur hexafluoride and phosphorus pentachloride. If all the phosphorus-chlorine bonds in a molecule are covalent, it would imply that the phosphorus molecule is violating the octet rule by holding a total of 10 valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




