
How will you obtain \[{\rm{Butan}} - 2 - {\rm{ol}}\] from
i) Propanal
ii) \[{\rm{Butan}} - 2 - {\rm{one}}\]
iii) \[{\rm{Butan}} - 2 - {\rm{ene}}\]
Answer
517.5k+ views
Hint: \[{\rm{Butan}} - 2 - {\rm{ol}}\] is a secondary alcohol i.e., butane is substituted by a hydroxy group at second position, which appears as colorless liquid having an alcoholic odor. The vapors of the given alcohol are heavier than the vapors of air. It is easily soluble in water.
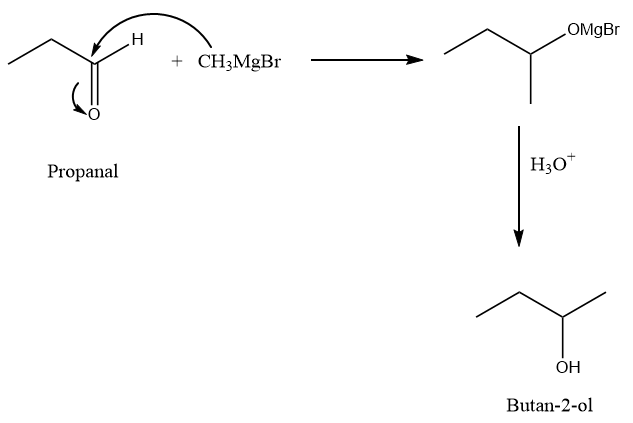
Complete answer: Preparation of \[{\rm{Butan}} - 2 - {\rm{ol}}\] from Propanal:
When Propanal is reacted with the Grignard reagent, the methyl group acts as a nucleophile and attacks at the carbonyl center of the compound due to which formation of an intermediate takes place. Later on, reaction with water in the acidic medium converts the intermediate compound into \[{\rm{Butan}} - 2 - {\rm{ol}}\]. The reaction proceeds as follows:
Preparation of \[{\rm{Butan}} - 2 - {\rm{ol}}\] from \[{\rm{Butan}} - 2 - {\rm{one}}\]:
The ketone group of \[{\rm{Butan}} - 2 - {\rm{one}}\] on reduction in the presence of lithium aluminium hydride and ether, converts into a hydroxyl group. Hence formation of \[{\rm{Butan}} - 2 - {\rm{ol}}\] takes place. The reaction proceeds as follows:
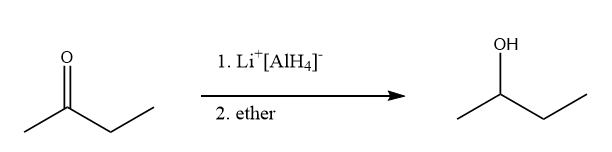
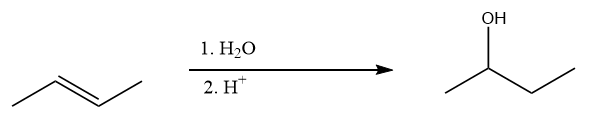
Preparation of \[{\rm{Butan}} - 2 - {\rm{ol}}\] to \[{\rm{Butan}} - 2 - {\rm{ene}}\]:
On hydrolysis of \[{\rm{Butan}} - 2 - {\rm{ene}}\]in the presence of an acid, the reaction follows Markovnikov’s electrophilic addition i.e., electronegative part will be bonded to the more substituted carbon atom. Hence, the hydroxyl group which is an electronegative part of water will attack second carbon of given unsaturated alkene and formation of \[{\rm{Butan}} - 2 - {\rm{ol}}\] takes place. The reaction proceeds as follows:
Note:
On bromination of Butane in the presence of light, \[2 - {\rm{bromobutane}}\]is formed which is an isomer of monobromo derivative of butane. Then, on reacting \[2 - {\rm{bromobutane}}\] with \[NaOH\], removal of sodium chloride takes place and a hydroxyl group is introduced at the second carbon of butane. Hence, \[{\rm{Butan}} - 2 - {\rm{ol}}\] can alternatively be prepared from Butane.
Complete answer: Preparation of \[{\rm{Butan}} - 2 - {\rm{ol}}\] from Propanal:
When Propanal is reacted with the Grignard reagent, the methyl group acts as a nucleophile and attacks at the carbonyl center of the compound due to which formation of an intermediate takes place. Later on, reaction with water in the acidic medium converts the intermediate compound into \[{\rm{Butan}} - 2 - {\rm{ol}}\]. The reaction proceeds as follows:

Preparation of \[{\rm{Butan}} - 2 - {\rm{ol}}\] from \[{\rm{Butan}} - 2 - {\rm{one}}\]:
The ketone group of \[{\rm{Butan}} - 2 - {\rm{one}}\] on reduction in the presence of lithium aluminium hydride and ether, converts into a hydroxyl group. Hence formation of \[{\rm{Butan}} - 2 - {\rm{ol}}\] takes place. The reaction proceeds as follows:
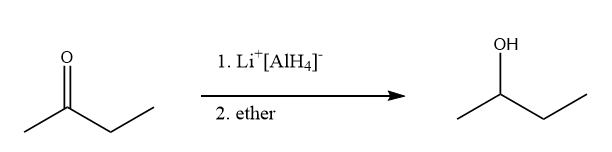
Preparation of \[{\rm{Butan}} - 2 - {\rm{ol}}\] to \[{\rm{Butan}} - 2 - {\rm{ene}}\]:
On hydrolysis of \[{\rm{Butan}} - 2 - {\rm{ene}}\]in the presence of an acid, the reaction follows Markovnikov’s electrophilic addition i.e., electronegative part will be bonded to the more substituted carbon atom. Hence, the hydroxyl group which is an electronegative part of water will attack second carbon of given unsaturated alkene and formation of \[{\rm{Butan}} - 2 - {\rm{ol}}\] takes place. The reaction proceeds as follows:

Note:
On bromination of Butane in the presence of light, \[2 - {\rm{bromobutane}}\]is formed which is an isomer of monobromo derivative of butane. Then, on reacting \[2 - {\rm{bromobutane}}\] with \[NaOH\], removal of sodium chloride takes place and a hydroxyl group is introduced at the second carbon of butane. Hence, \[{\rm{Butan}} - 2 - {\rm{ol}}\] can alternatively be prepared from Butane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




