
How will you obtain p-chlorobenzaldehyde from benzene?
Answer
509.4k+ views
Hint: Electrophilic aromatic substitution reaction: It is an organic reaction in which an electrophilic group (generally hydrogen atom) gets replaced with an electrophile when reacted in the presence of required reagent. Some examples of electrophilic substitution reactions are halogenation, nitration, Friedel craft alkylation etc.
Complete answer:
For the given conversion, the following steps are involved in the reaction mechanism:
Step-1: The benzene molecule undergoes electrophilic aromatic substitution reaction i.e., halogenation reaction in the presence of $ B{r_2} $ and $ FeB{r_3} $ . The reaction proceeds as follows:
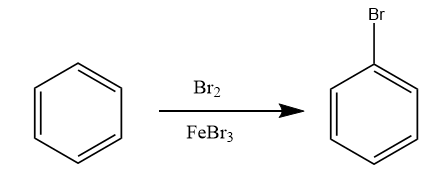
Step-2: The bromobenzene further reacts with magnesium metal in the presence of ether to form Grignard reagent of type $ R - Mg - X $ . The reaction proceeds as follows:
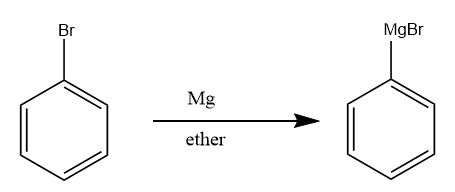
Step-3: The Grignard reagent formed, reacts with formaldehyde in the acidic medium to form the benzyl alcohol. The reaction takes place as follows:
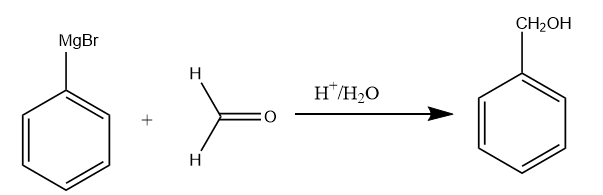
Step-4: The benzyl alcohol again undergoes a halogenation reaction in the presence of $ C{l_2} $ and $ FeC{l_3} $ . As there is an alkyl group present on the benzene which is ortho-para directing, so the formation of para chlorobenzyl alcohol will take place. The reaction proceeds as follows:

Step-5: Now the alcoholic group of benzyl alcohol is oxidized when para chlorobenzyl alcohol reacts with chromium trioxide in the presence of base like pyridine. Then, the final product is obtained i.e., para chlorobenzaldehyde. The reaction takes place as follows:
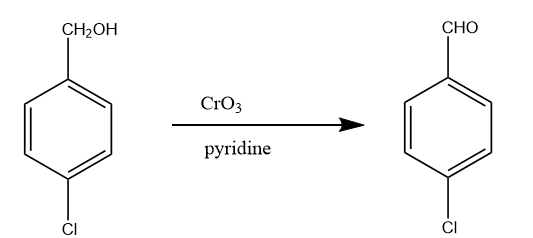
Note:
It is important to note that in this conversion, we cannot first substitute the chlorine group to the benzene ring because it is a good leaving group and it will eventually be replaced and the benzene ring will tend to show nucleophilic substitution reaction under specific conditions.
Complete answer:
For the given conversion, the following steps are involved in the reaction mechanism:
Step-1: The benzene molecule undergoes electrophilic aromatic substitution reaction i.e., halogenation reaction in the presence of $ B{r_2} $ and $ FeB{r_3} $ . The reaction proceeds as follows:

Step-2: The bromobenzene further reacts with magnesium metal in the presence of ether to form Grignard reagent of type $ R - Mg - X $ . The reaction proceeds as follows:

Step-3: The Grignard reagent formed, reacts with formaldehyde in the acidic medium to form the benzyl alcohol. The reaction takes place as follows:

Step-4: The benzyl alcohol again undergoes a halogenation reaction in the presence of $ C{l_2} $ and $ FeC{l_3} $ . As there is an alkyl group present on the benzene which is ortho-para directing, so the formation of para chlorobenzyl alcohol will take place. The reaction proceeds as follows:

Step-5: Now the alcoholic group of benzyl alcohol is oxidized when para chlorobenzyl alcohol reacts with chromium trioxide in the presence of base like pyridine. Then, the final product is obtained i.e., para chlorobenzaldehyde. The reaction takes place as follows:

Note:
It is important to note that in this conversion, we cannot first substitute the chlorine group to the benzene ring because it is a good leaving group and it will eventually be replaced and the benzene ring will tend to show nucleophilic substitution reaction under specific conditions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




