
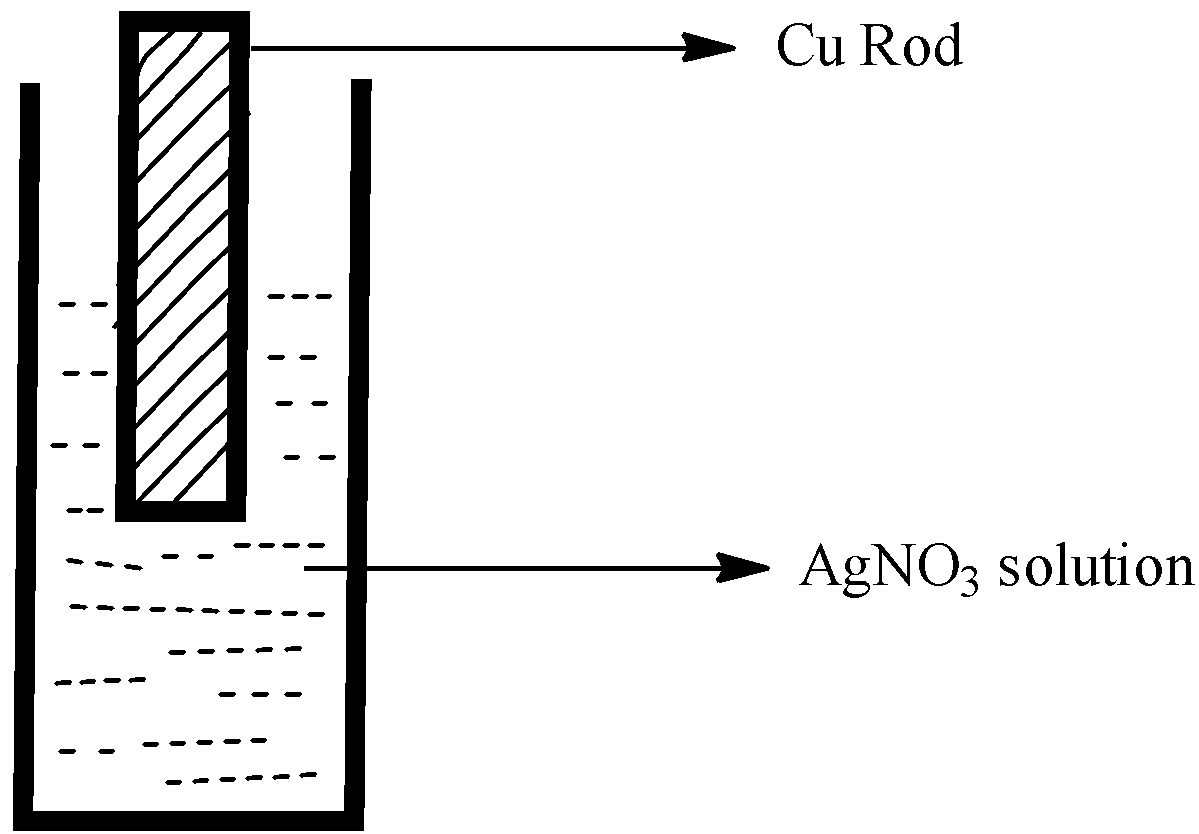
Observe the diagram showing a copper rod kept immersed in silver nitrate solution.
a. What is the colour change of the solution?
b. Write the balanced chemical equation for the reaction.

Answer
542.4k+ views
Hint: This reaction is based on the concept of displacement reaction. Compare the copper, and silver element according to the electrochemical series. The colour, and the reaction would be known.
Complete answer:
- First, by observing the diagram, we get to know that the copper rod is immersed in the silver nitrate solution, and it leads to the displacement reaction.
- Electrochemical series is the one which describes the arrangement of elements in order of their increasing electrode potential values.
- Now, let us answer part a. According to the electrochemical series, we know that copper is a more reactive element than the silver metal.
- So, when we immerse it in the silver nitrate solution, it displaces the silver leading to the formation of copper nitrate. The copper compounds mostly are blue, or green in colour.
- Thus, the colour of copper nitrate obtained is bluish-green.
- The colour change is from grey (colour of silver metal) to bluish green.
- Now, if we talk about part b. We have to write the balanced chemical equation.
- We already know it is a displacement reaction, in this reaction both oxidation, and reduction takes place.
- The oxidation number change happens like Cu (0) to Cu (+1), and Ag (+1) to Ag (0).
- Thus, the balanced chemical equation is
\[Cu(s)+2AgN{{O}_{3}}(aq)\to Cu{{(N{{O}_{3}})}_{2}}(aq)+2Ag(s)\]
- In the last, we can conclude that there is formation of copper nitrate solution leading to the colour change attained is bluish green.
Note:
Don’t get confused while writing the reaction. Just remember that this is a displacement reaction, and both oxidation, and reduction occurs in it. You must know the oxidation number represented by the elements, and most important the electrochemical series. So, we will get to know that copper metal is more reactive than the silver metal.
Complete answer:
- First, by observing the diagram, we get to know that the copper rod is immersed in the silver nitrate solution, and it leads to the displacement reaction.
- Electrochemical series is the one which describes the arrangement of elements in order of their increasing electrode potential values.
- Now, let us answer part a. According to the electrochemical series, we know that copper is a more reactive element than the silver metal.
- So, when we immerse it in the silver nitrate solution, it displaces the silver leading to the formation of copper nitrate. The copper compounds mostly are blue, or green in colour.
- Thus, the colour of copper nitrate obtained is bluish-green.
- The colour change is from grey (colour of silver metal) to bluish green.
- Now, if we talk about part b. We have to write the balanced chemical equation.
- We already know it is a displacement reaction, in this reaction both oxidation, and reduction takes place.
- The oxidation number change happens like Cu (0) to Cu (+1), and Ag (+1) to Ag (0).
- Thus, the balanced chemical equation is
\[Cu(s)+2AgN{{O}_{3}}(aq)\to Cu{{(N{{O}_{3}})}_{2}}(aq)+2Ag(s)\]
- In the last, we can conclude that there is formation of copper nitrate solution leading to the colour change attained is bluish green.
Note:
Don’t get confused while writing the reaction. Just remember that this is a displacement reaction, and both oxidation, and reduction occurs in it. You must know the oxidation number represented by the elements, and most important the electrochemical series. So, we will get to know that copper metal is more reactive than the silver metal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




