
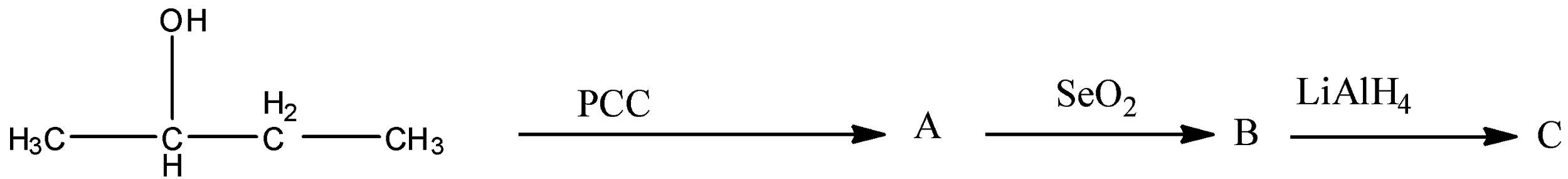
Number of possible stereoisomers in C
(1)2
(2)3
(3)4
(4)5

Answer
579.9k+ views
Hint: We know that isomers that possess different spatial atomic arrangement but same atomic connectivity are termed as stereoisomers. There are two types of stereoisomers, geometric isomers and optical isomers.
Complete step by step answer:
Here, first we have to complete the reaction and then we have to find out the stereoisomers of C.
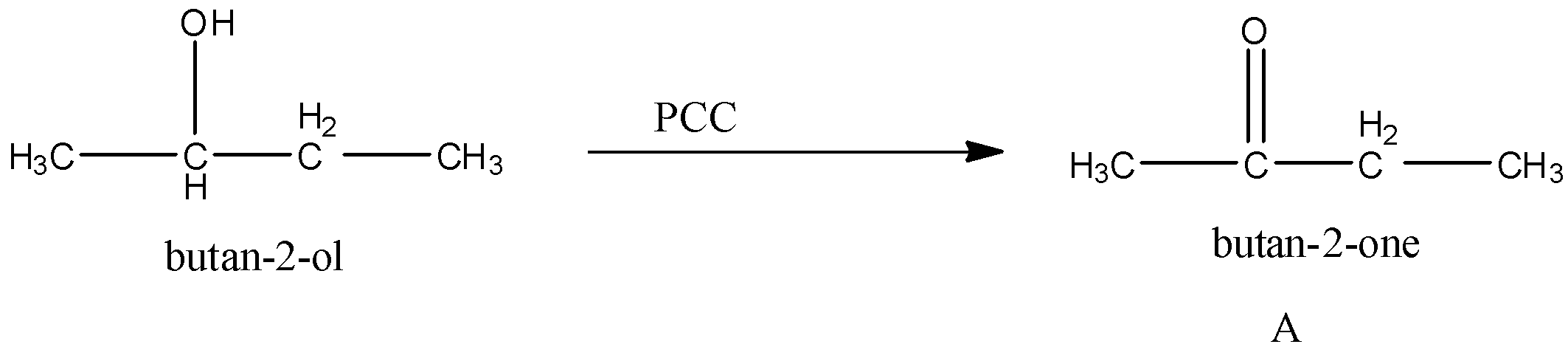
First the butan-2-ol reacts with PCC. We know that PCC is an oxidizing agent. It converts alcohol to carbonyl groups. So, the product A formed is,
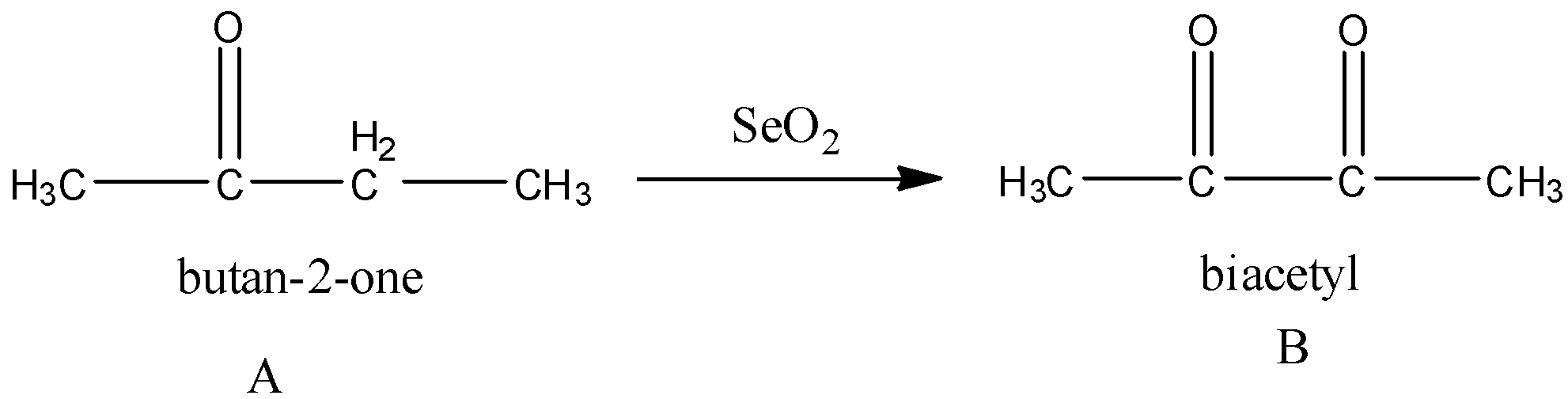
Now, A (butan-2-one) reacts with ${\rm{Se}}{{\rm{O}}_{\rm{2}}}$. Selenium dioxide converts an active methylene group to a carbonyl group. Methylene group is the carbon atom that is bonded to two hydrogen atoms.
Next, B reacts with ${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$. We know that lithium aluminium hydride $\left( {{\rm{LiAl}}{{\rm{H}}_{\rm{4}}}} \right)$ is a reducing agent. It can reduce ketone, carboxylic acid, aldehyde to alcohol groups.
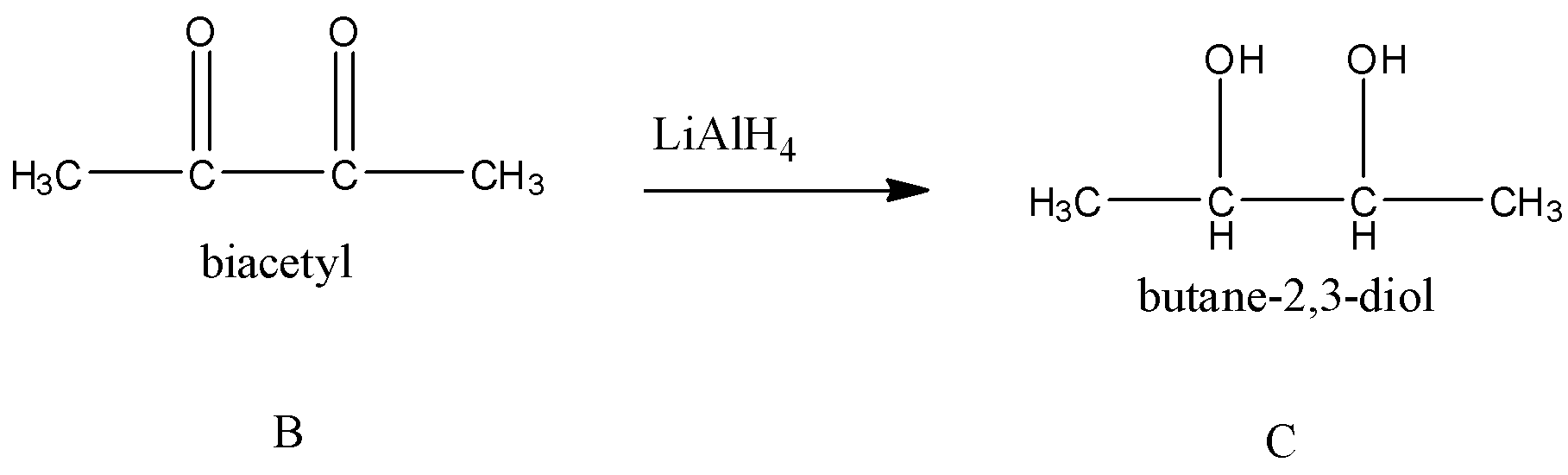
So, the compound C formed is butane-2,3-diol. Now, we have to find out the number of isomers for butane-2,3-diol.
We know the formula to calculate the number of stereoisomers is ${2^n}$. Here, n represents the number of chiral centres.
In butane-2,3 diol, two chiral centres are present.
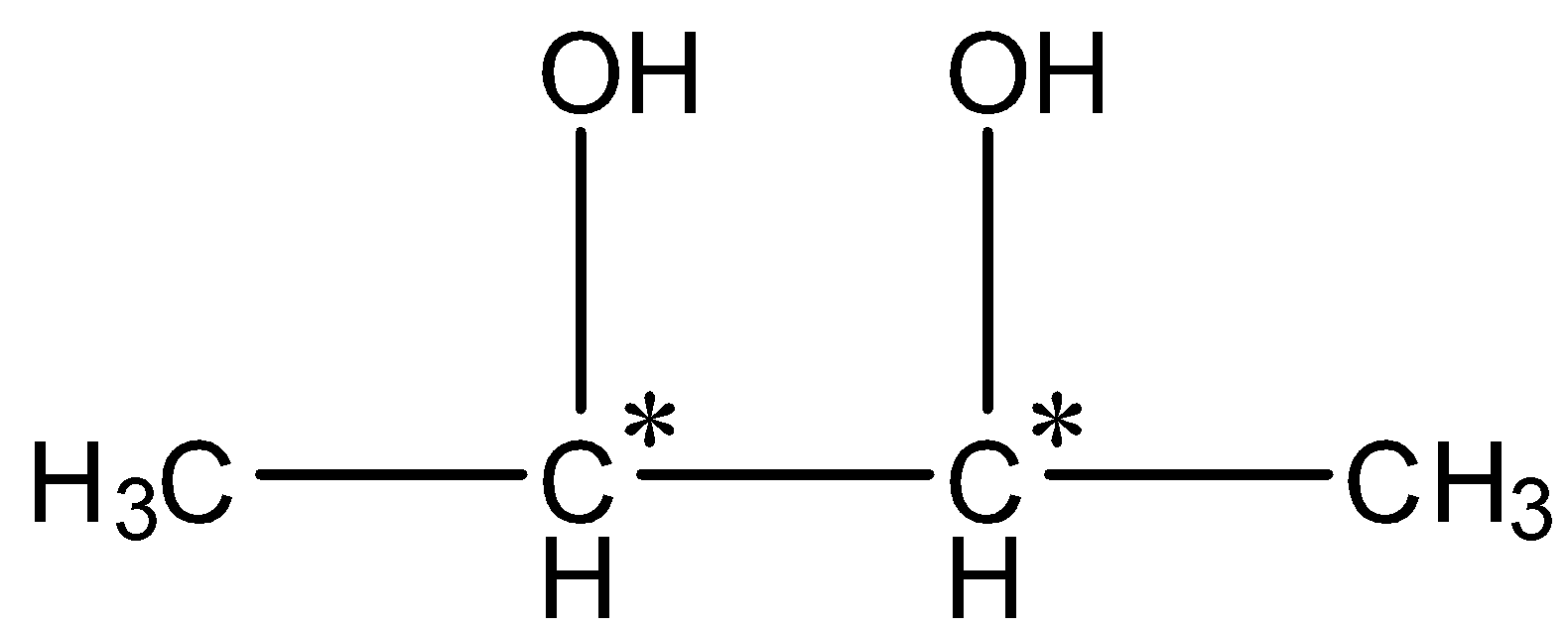
So, the number of stereoisomers=${2^2} = 4$. Therefore, the number of stereoisomers is 4.
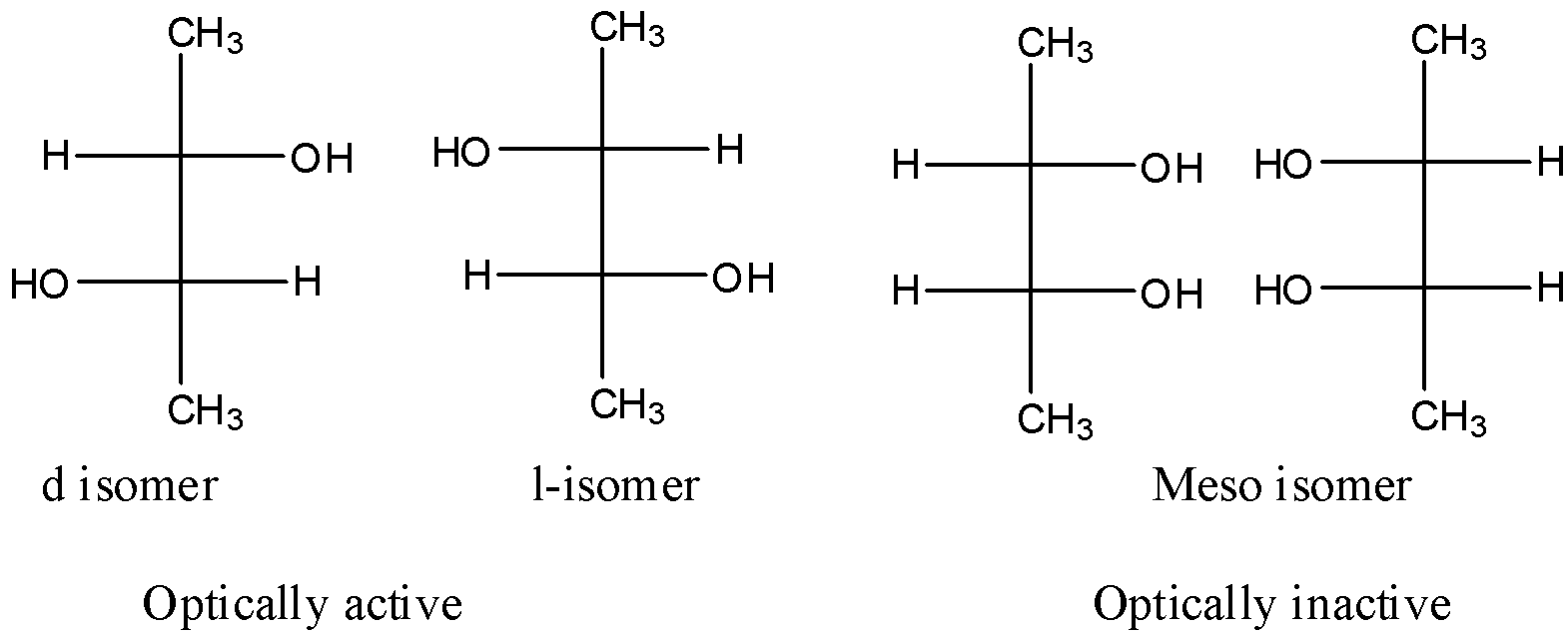
Hence, the correct answer is C.
Note:
Always remember that a compound can have stereoisomers only if it possesses chiral centres. Chiral central is the atom in which all the four groups attached are different. To identify whether an atom is chiral or not, we have to look at the groups bonded to it. If all the bonded groups are different it indicates that the atom is a chiral centre.
Complete step by step answer:
Here, first we have to complete the reaction and then we have to find out the stereoisomers of C.
First the butan-2-ol reacts with PCC. We know that PCC is an oxidizing agent. It converts alcohol to carbonyl groups. So, the product A formed is,

Now, A (butan-2-one) reacts with ${\rm{Se}}{{\rm{O}}_{\rm{2}}}$. Selenium dioxide converts an active methylene group to a carbonyl group. Methylene group is the carbon atom that is bonded to two hydrogen atoms.

Next, B reacts with ${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$. We know that lithium aluminium hydride $\left( {{\rm{LiAl}}{{\rm{H}}_{\rm{4}}}} \right)$ is a reducing agent. It can reduce ketone, carboxylic acid, aldehyde to alcohol groups.

So, the compound C formed is butane-2,3-diol. Now, we have to find out the number of isomers for butane-2,3-diol.
We know the formula to calculate the number of stereoisomers is ${2^n}$. Here, n represents the number of chiral centres.
In butane-2,3 diol, two chiral centres are present.

So, the number of stereoisomers=${2^2} = 4$. Therefore, the number of stereoisomers is 4.

Hence, the correct answer is C.
Note:
Always remember that a compound can have stereoisomers only if it possesses chiral centres. Chiral central is the atom in which all the four groups attached are different. To identify whether an atom is chiral or not, we have to look at the groups bonded to it. If all the bonded groups are different it indicates that the atom is a chiral centre.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




