
Number of pi bonds in \[Xe{O_4}\]is:
Answer
569.4k+ views
Hint: The structure of xenon compound reveals the number of pi bonds present. The hybridization of xenon compounds determines the number of bonds present in between xenon and oxygen atoms.
Complete step by step answer: \[Xe{O_4}\] is the compound formed of xenon and fluorine atoms. Xenon is the central atom in \[Xe{O_4}\].
\[Xe{O_4}\] is synthesized by treating sodium or barium xenate with conc.\[{H_2}S{O_4}\]. Sodium and barium xenates have chemical formulas as \[N{a_4}Xe{O_6}\] and\[B{a_2}Xe{O_6}\] . The \[Xe{O_4}\] is purified using sublimation at\[195K\] .
$N{a_4}Xe{O_6} + 2{H_2}S{O_4} \to Xe{O_4} + 2N{a_2}S{O_4} + 2{H_2}O$
$B{a_2}Xe{O_6} + 2{H_2}S{O_4} \to Xe{O_4} + 2BaS{O_4} + 2{H_2}O$
In order to find the number of pi bonds in \[Xe{O_4}\], the number of structures has to be determined first. For this the VSEPR theory is used. Xenon is an element in the periodic table with atomic number \[54\] and electronic configuration is\[\left[ {Kr} \right]4{d^{10}}5{s^2}5{p^6}\] .
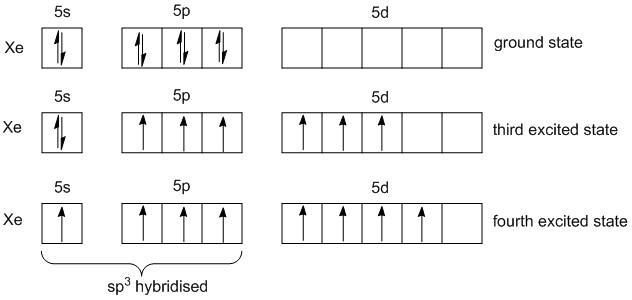
The number of valence electrons of Xenon in ground state is \[8\]. In the fourth excited, xenon atom has \[8\] unpaired electrons. One electron in s-orbital and three electrons in p-orbitals undergo \[s{p^3}\] hybridization.
As no lone pair of electrons is present the shape of \[Xe{O_4}\] is tetrahedral with the bond angle of\[{109^o}28'\]. The structure of the \[Xe{O_4}\] reveals that there are four sigma bonds and four pi bonds. The s and p orbitals are involved in sigma bonds and the d-orbitals are involved in pi bonds.The pi bonds are formed between the p-orbitals of oxygen and the d-orbitals of xenon. Thus they are also labeled as \[p\pi - d\pi \] bonds.
Note:
Xenon is a noble gas but it forms compounds in excited states. The outer 5d orbitals are used for pi bond formation.
Complete step by step answer: \[Xe{O_4}\] is the compound formed of xenon and fluorine atoms. Xenon is the central atom in \[Xe{O_4}\].
\[Xe{O_4}\] is synthesized by treating sodium or barium xenate with conc.\[{H_2}S{O_4}\]. Sodium and barium xenates have chemical formulas as \[N{a_4}Xe{O_6}\] and\[B{a_2}Xe{O_6}\] . The \[Xe{O_4}\] is purified using sublimation at\[195K\] .
$N{a_4}Xe{O_6} + 2{H_2}S{O_4} \to Xe{O_4} + 2N{a_2}S{O_4} + 2{H_2}O$
$B{a_2}Xe{O_6} + 2{H_2}S{O_4} \to Xe{O_4} + 2BaS{O_4} + 2{H_2}O$
In order to find the number of pi bonds in \[Xe{O_4}\], the number of structures has to be determined first. For this the VSEPR theory is used. Xenon is an element in the periodic table with atomic number \[54\] and electronic configuration is\[\left[ {Kr} \right]4{d^{10}}5{s^2}5{p^6}\] .

The number of valence electrons of Xenon in ground state is \[8\]. In the fourth excited, xenon atom has \[8\] unpaired electrons. One electron in s-orbital and three electrons in p-orbitals undergo \[s{p^3}\] hybridization.
As no lone pair of electrons is present the shape of \[Xe{O_4}\] is tetrahedral with the bond angle of\[{109^o}28'\]. The structure of the \[Xe{O_4}\] reveals that there are four sigma bonds and four pi bonds. The s and p orbitals are involved in sigma bonds and the d-orbitals are involved in pi bonds.The pi bonds are formed between the p-orbitals of oxygen and the d-orbitals of xenon. Thus they are also labeled as \[p\pi - d\pi \] bonds.
Note:
Xenon is a noble gas but it forms compounds in excited states. The outer 5d orbitals are used for pi bond formation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




