
Number of peroxide bonds in ${{K}_{3}}Cr{{O}_{8}}$ is 4.
If true enter 1, else enter 0.
Answer
586.2k+ views
Hint: Check if peroxide bonds are present. Find the oxidation state of chromium through a unitary method of balancing charges. If this value is greater than the maximum oxidation state shown by chromium state, we can say that peroxide bonds are present. Draw the expanded structure of the compound to find the number of peroxide bonds present.
Complete step-by-step answer:
Peroxides are a group of compounds with the structure $R-O-O-R$. The $O-O$ group in a peroxide is called peroxy or peroxide group. The oxidation of oxygen in peroxy bonds is -1 which is different from its usual oxidation state of -2.
There are different types of peroxides, namely:
- Peroxy acids that are derivative of carboxylic acids,
- Metal peroxides like $Ba{{O}_{2}}$,$N{{a}_{2}}{{O}_{2}}$,
- Organic peroxides like butyl hydroperoxide,
- Main group peroxide like potassium peroxydisulfate.
Peroxides is used as a strong oxidising agent and bleaching agent. This is because peroxide releases nascent oxygen.
As suggested in the hint, we will draw the structure of the ${{K}_{3}}Cr{{O}_{8}}$:
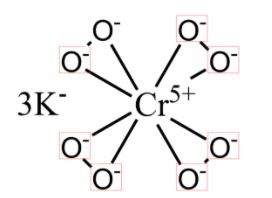
In the above structure we observe that there are 4 $O-O$ bonds i.e. 4 peroxy bonds. Therefore, the statement number of peroxide bonds in ${{K}_{3}}Cr{{O}_{8}}$ is 4 is true.
Note: Potassium peroxochromate is an inorganic chemical which is reddish brown in colour. The compound is paramagnetic. It is a unique compound as it is one of the few compounds in which chromium exists in +5 oxidation state. The compound is mainly stabilised peroxide ligands.
Complete step-by-step answer:
Peroxides are a group of compounds with the structure $R-O-O-R$. The $O-O$ group in a peroxide is called peroxy or peroxide group. The oxidation of oxygen in peroxy bonds is -1 which is different from its usual oxidation state of -2.
There are different types of peroxides, namely:
- Peroxy acids that are derivative of carboxylic acids,
- Metal peroxides like $Ba{{O}_{2}}$,$N{{a}_{2}}{{O}_{2}}$,
- Organic peroxides like butyl hydroperoxide,
- Main group peroxide like potassium peroxydisulfate.
Peroxides is used as a strong oxidising agent and bleaching agent. This is because peroxide releases nascent oxygen.
As suggested in the hint, we will draw the structure of the ${{K}_{3}}Cr{{O}_{8}}$:

In the above structure we observe that there are 4 $O-O$ bonds i.e. 4 peroxy bonds. Therefore, the statement number of peroxide bonds in ${{K}_{3}}Cr{{O}_{8}}$ is 4 is true.
Note: Potassium peroxochromate is an inorganic chemical which is reddish brown in colour. The compound is paramagnetic. It is a unique compound as it is one of the few compounds in which chromium exists in +5 oxidation state. The compound is mainly stabilised peroxide ligands.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




