
Number of isomers of hexane
Answer
600.9k+ views
Hint: To correctly tell the answer of the question, we should know that isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space.
Step by step solution:
We should know that Hexane is a straight-chain alkane with six carbon atoms and has the molecular formula C6H14. We should also know that isomers have the same molecular formula, but have a different arrangement of the atoms in space. We should know that in structural isomerism, the atoms are arranged in a completely different order.
Now, we will draw isomers of hexane. We should follow these important points to draw correct isomers. We should first identify and count all the atoms which are to be drawn in the isomers. This will give us a molecular formula. We should know that any isomers drawn will contain the same number of each type of atom found in the original molecular formula of the compound. So, let us draw the isomers of hexane.
Note:
We use hexanes in industries for formation of glues for shoes, leather products. We use hexane to extract oils from seeds, for cleansing and degreasing a variety of items, and in textile manufacturing. We should know that hexanes are chiefly obtained by refining crude oil. It is important to note that like most alkanes, hexane characteristically exhibits low reactivity and are suitable solvents for reactive compounds. We should note that acute (short-term) inhalation exposure of humans to high levels of hexane causes mild central nervous system (CNS) effects, including dizziness, giddiness, slight nausea, and headache.
Step by step solution:
We should know that Hexane is a straight-chain alkane with six carbon atoms and has the molecular formula C6H14. We should also know that isomers have the same molecular formula, but have a different arrangement of the atoms in space. We should know that in structural isomerism, the atoms are arranged in a completely different order.
Now, we will draw isomers of hexane. We should follow these important points to draw correct isomers. We should first identify and count all the atoms which are to be drawn in the isomers. This will give us a molecular formula. We should know that any isomers drawn will contain the same number of each type of atom found in the original molecular formula of the compound. So, let us draw the isomers of hexane.
| Common name | IUPAC name | Text formula | Skeletal formula |
| normal hexane n-hexane | hexane | \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\] | 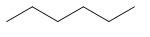
|
| Iso-hexane | 2-methylpentane | \[{{(C{{H}_{3}})}_{2}}CH{{(C{{H}_{2}})}_{2}}C{{H}_{3}}\] | 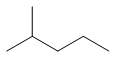
|
| 3-methylpentane | \[C{{H}_{3}}C{{H}_{2}}CH(C{{H}_{3}})C{{H}_{2}}C{{H}_{3}}\] | 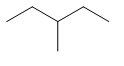
| |
| 2,3-dimethylbutane | \[{{(C{{H}_{3}})}_{2}}CHCH{{(C{{H}_{3}})}_{2}}\] | 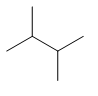
| |
| neohexane | 2,2-dimethylbutane | \[{{(C{{H}_{3}})}_{3}}CC{{H}_{2}}C{{H}_{3}}\] | 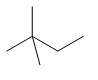
|
Note:
We use hexanes in industries for formation of glues for shoes, leather products. We use hexane to extract oils from seeds, for cleansing and degreasing a variety of items, and in textile manufacturing. We should know that hexanes are chiefly obtained by refining crude oil. It is important to note that like most alkanes, hexane characteristically exhibits low reactivity and are suitable solvents for reactive compounds. We should note that acute (short-term) inhalation exposure of humans to high levels of hexane causes mild central nervous system (CNS) effects, including dizziness, giddiness, slight nausea, and headache.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




