
What is not true regarding the products?
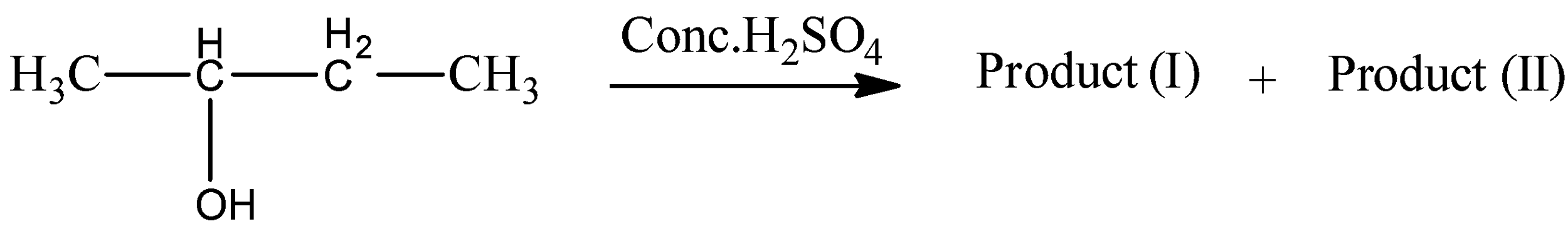
A. Product-I and II are position isomers
B. Product-I and II contains the same number of $s{p^3}$ and $s{p^2}$ carbon atoms
C. The yield of the product I and product II is same
D. Reaction obeys Saytzeff rule

Answer
504k+ views
Hint: We have to know that, the drying out response of alcohols to produce alkene continues by warming the alcohols within the sight of a solid corrosive, like sulfuric or phosphoric corrosive, at high temperatures.
Complete answer:
The given alcohol compound is $bu\tan - 2 - ol$ . This $bu\tan - 2 - ol$ reacts with concentrated hydrochloric acid to give product (I) alkene and product (II) alkene.
At the point when you get dried out a liquor, you eliminate the - OH bunch, and a hydrogen iota from the following carbon particle in the chain. With atoms like $bu\tan - 2 - ol$ , there are two prospects when that occurs.
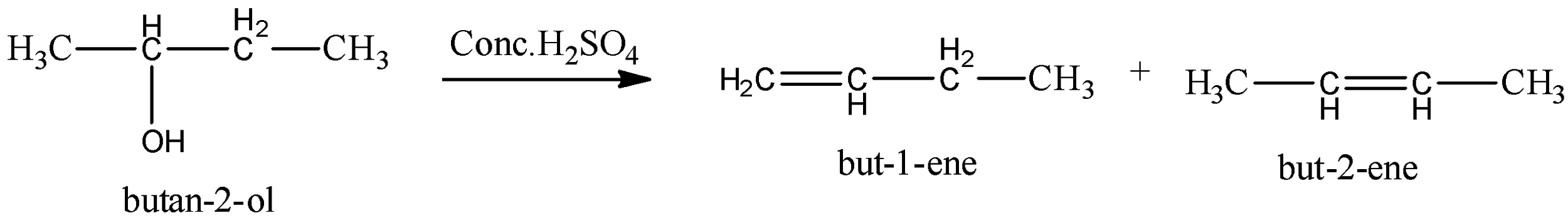
Drying out of butan-2-ol prompts a blend containing:
$but - 1 - ene$
$cis - but - 2 - ene$ (otherwise called $\left( Z \right) - but - 2 - ene$ )
$trans - but - 2 - ene$ (otherwise called $\left( E \right) - but - 2 - ene$ ).
Alcohols go through lack of hydration (loss of water particle) in acidic medium to give olefins. A twofold bond is shaped because of the loss of water particles. It's anything but an end-response. As indicated by Saytzeff's standard (likewise Zaitsev's standard), during lack of hydration, more subbed alkene (olefin) is shaped as a significant item, since more prominent the replacement of twofold bond more noteworthy is the dependability of alkene.
Therefore, option (C) is not true.
Note:
Consider the longest chain containing the twofold bond: If two gatherings (connected to the carbons of the twofold bond) are on a similar side of the twofold bond, the isomer is a cis alkene. On the off chance that the two gatherings lie on inverse sides of the twofold bond, the isomer is a trans alkene.
Complete answer:
The given alcohol compound is $bu\tan - 2 - ol$ . This $bu\tan - 2 - ol$ reacts with concentrated hydrochloric acid to give product (I) alkene and product (II) alkene.
At the point when you get dried out a liquor, you eliminate the - OH bunch, and a hydrogen iota from the following carbon particle in the chain. With atoms like $bu\tan - 2 - ol$ , there are two prospects when that occurs.
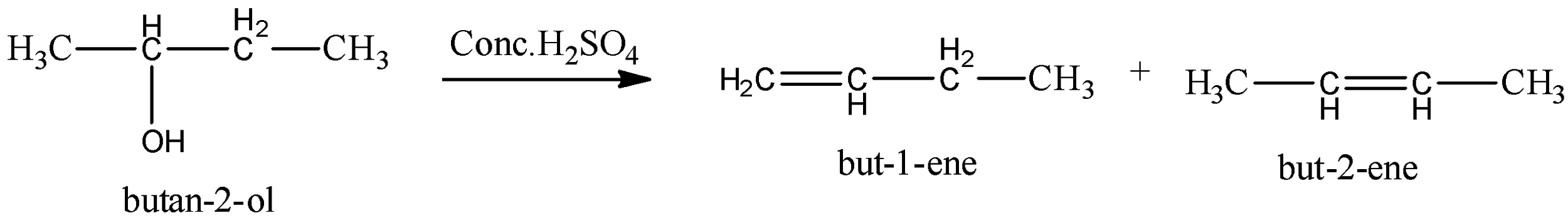
Drying out of butan-2-ol prompts a blend containing:
$but - 1 - ene$
$cis - but - 2 - ene$ (otherwise called $\left( Z \right) - but - 2 - ene$ )
$trans - but - 2 - ene$ (otherwise called $\left( E \right) - but - 2 - ene$ ).
Alcohols go through lack of hydration (loss of water particle) in acidic medium to give olefins. A twofold bond is shaped because of the loss of water particles. It's anything but an end-response. As indicated by Saytzeff's standard (likewise Zaitsev's standard), during lack of hydration, more subbed alkene (olefin) is shaped as a significant item, since more prominent the replacement of twofold bond more noteworthy is the dependability of alkene.
Therefore, option (C) is not true.
Note:
Consider the longest chain containing the twofold bond: If two gatherings (connected to the carbons of the twofold bond) are on a similar side of the twofold bond, the isomer is a cis alkene. On the off chance that the two gatherings lie on inverse sides of the twofold bond, the isomer is a trans alkene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




